Morphological
Characterization and Evaluation of Sorghum [Sorghum
bicolor (L.) Moench]
Landraces in Benishangul Gumuz, North-western
Ethiopia
Gedifew Gebrie (MSc)1 and Tsige Genet (PhD)2
Department of Plant Science, Faculty of Agricultural and
Environmental Sciences, Bahir Dar University, Bahir Dar, Ethiopia
|
ARTICLE
INFO
|
ABSTRACT
|
|
Article No.: 123118187
Type: Research
DOI: 10.15580/GJAS.2019.1.123118187
|
Although sorghum is an important cereal
crop in Benishangul Gumuz, few studies have been undertaken on existing
diversity. Hence, the objective of this study was to characterize sorghum
landraces collected from five districts of north western BGRS. Twenty-five
sorghum genotypes including the local check (Emohay) were tested in RCBD with
three replications during the 20011/2012 rainy seasons at Pawe to assess the
presence and degree of variability for desired morphological traits in
sorghum landraces. Plots with two rows per plot each 5 m long with spacing of
0.75 m between rows, 0.15 m between plants and 1.5 m between blocks (a plot
area of 7.5 m2) was used. Analysis of variance revealed
significant differences among entries for most quantitative characters.
Eleven landraces gave better grain yield between 41.38 to 48.9 q/ha
indicating the possibility of identifying superior genotype to be selected
among the landraces. Genotype 021pw-2010 was the highest yielded (48.9 q/ha)
landrace. The lowest GCV and PCV were obtained for
days to maturity while the highest PCV and GCV were obtained for plant
height. Higher ratios of the phenotypic variance to genotypic variance were recorded
for days to flowering, days to maturity, leaf length, leaf width, single leaf
area, number of leaves, plant height, head length, head width, head weight
and grain yield indicating that the traits will be highly influenced by the
environment. Broad sense heritability estimates for the traits ranged from
45.1% (for head weight) to 99.8% (for single leaf area). Grain yield showed
higher significant positive genetic correlation with days to maturity, head
weight, head width and head length, and a negative significant correlation
with single leaf area and number of leaves at phenotypic level. The high
performing accessions of the landraces screened in this study should further
be evaluated under a wide range of environments to find widely adapting landraces.
The variability observed in the study could be given emphasis while planning
a breeding strategy for increased grain yield. Collection, conservation and
utilization of available materials across BGRS must be given attention.
|
|
Submitted: 31/12/2018
Accepted: 11/01/2019
Published: 31/01/2019
|
|
*Corresponding
Author
Gedifew Gebrie
E-mail: gebriegedifew.y@ yahoo.com
Phone: (+251)946 45 44 37
|
|
Keywords: Variability; Heritability; Genetic Advance; Correlation; Path analysis.
|
|
|
|
1.
INTRODUCTION
Sorghum is a leading cereal crop in the arid and semi-arid
regions of the world, ranking fifth in importance among the world’s grain crops
after wheat, maize, rice and barley. It is a C4 crop particularly
adapted to the drought prone areas with hot, semi-arid tropical environments,
receiving an annual rainfall of 400-600 mm which is too dry for other cereals
like maize (Vittal et al., 2010). In
Ethiopia, it is an important food crop and is widely grown in the high land,
low land and semi-arid region of the country (Adugna, 2007). It is a
traditional food widely grown in Ethiopia, in 13 of the 18 major agro
ecological zones, grown on over 1.3 million hectares. It is predominantly used
for food, 80 percent of it for injera (flat pancake like traditional bread) making
following tef [Eragrostis tef (Zucc.)
Trotter] (Yilma, 1991).
As one of the leading traditional
food cereals in Ethiopia, in terms of both total production and area, major
research efforts have been directed towards the improvement and stabilization of
sorghum yields. At a national level, sorghum improvement involves the
manipulation of indigenous and introduced germplasm to develop adapted types
for the various ecological zones (Yilma, 1991). Ethiopia has a diverse wealth
of sorghum germplasm adapted to a range of altitudes and rainfall conditions.
Because of its global socio-economic importance, there is a constant need for
the improvement of sorghum (Vittal et al.,
2010). There is high genetic diversity in sorghum in Ethiopia with several
pockets of geographical isolation. The current number of indigenous sorghum germplasm
contained in the gene bank stands at about 6 thousand and represents a wide
array of diversity in the major sorghum growing areas of Ethiopia. This could
serve as valuable genetic base for breeding and improvement of the crop in the
country and the world at large (Wasihun, 2007).
Highly
desirable genetic characteristics were identified in some of the Ethiopian
sorghum germplasm and were utilized in breeding programs extensively elsewhere
in the world. The wealth of genetic
variability in the Ethiopian germplasm has been and will continue to be useful
sources of economically important traits (Melaku, 1988). Important traits
reported from Ethiopian sorghum include cold tolerance, drought resistance,
resistance to sorghum shoot fly, disease and pest resistance, grain quality and
resistance to grain mould, high sugar content in the stalks, and high lysine
and protein content (IBC, 2008).
Systematic characterizations and evaluation of plant genetic resources
are prerequisites for the efficient use of material through conventional
methods. Morphological, biochemical and molecular procedures are currently
being employed in evaluating plant genetic resources (Mahmoods
et al., 2008). It was found earlier
that genetic improvement of crops for quantitative traits requires reliable
estimates of genetic variability, heritability and genetic advancement in
respect to the breeding material that is presently at hand in order to plan an
efficient breeding program. And the information on variability and heritability
of characters is essential for identifying characters amenable to genetic
improvement through selection (Govindaraj
et al., 2010).
Although the
diversity of sorghum is high in Ethiopia, the extent and distribution of
genetic variability of the crop in different ecologies has not been properly
studied (Melakhail, 1975). One of the
potential areas is the north western Benishangul Gumuz regional state of
Ethiopia. This zone lies within the area of sorghum domestication and as such
is rich in genetic diversity of cultivated sorghum and its wild forms. Because
of its unique agro ecological setting of warm humid climate, the area is rich
in special class of sorghum germplasm adapted to this condition. With the
intention of getting varieties, which fit to the area, a collection of landraces
was made from the four districts of Metekel zone. This study was conducted in
order to measure the level of variation in morphological characteristics of the
sorghum germplasm collections of this area.
The
objectives of the study were to:
1.
Determine the extent and degree of
variability in morpho-agronomic characters of the
sorghum collections
2.
Evaluate yield and yield associated traits
among the sorghum collections
3.
Identify the sorghum accessions that are
superior in desirable agronomic characters for use in the breeding programs.
The experiment was conducted
at PARC in Pawe district, Metekel Zone, north western part of Benishangul Gumuz
Regional State, Ethiopia, from mid-2011 to early 2012 main cropping rainy
season. The research center is located
at about 580 km north west of Addis Ababa and 230 km from Bahir dar at 36o25`E
longitude, 11o12'N latitude and at an altitude of 1150 meters above
sea level. The area is characterized by hot humid conditions with means maximum
and minimum temperatures of 32oc and 16oc
respectively. The annual rainfall ranges
from 1500-1800 mm with five and half months’ duration. The total rainfall
during the growing season of sorghum is about 1659 mm.
The soil
type is Haplic Altisols, very deep (>150 cm) and clay in
texture. The PH of the soil ranges from 5.5 to 6.9 and subsurface
soils have higher PH values than surface soils. The organic carbon (0.2
to 2.8 %) and total nitrogen (0.02 to 0.19 %) contents of the soil decrease
with soil depth. The cation exchange capacity ranges from 20 to 51cmolc/kg.
Twenty-five
accessions and one standard check (Table 1) were included in this study though
accession 032pw-2010 did not emerge in. The accessions were received from PARC. The local check variety is a released
commercial cultivar recommended for high and intermediate altitude zones in the
country.
The experimental
design was RCBD with three replications. Each genotype was planted in a plot
size of 7.5 m2 (2 rows, each 5 m long, with a spacing of 0.75 m
between rows and 0.15 m between plants). The spacing between blocks was 1.5 m.
The total net area requirement of the experiment was 729 m2. During planting the seeds were drilled in rows
and at about 20 days after planting thinned out to 0.15m distances. DAP (100 kg/ha)
and Urea (50 kg/ha) fertilizers were applied at planting and at knee height,
respectively.
Measurements and observations were recorded
following the IBPGR and ICRISAT (1993) descriptor list. Qualitative traits were
scored based on arbitrary scales found in the sorghum descriptors list as
reference for the observations. All quantitative data
were measured by taking the mean value of five plants, which were tagged
randomly before the time of data collection.
2.4.1.
Data collected on plant bases
a) Numbers of
leaves per plant - leaves were counted from the base to the flag leaf after the
time of blooming.
b) Leaf
length (cm) - the distance from the collar to the leaf tip of the third leaf
from the top was measured in cm.
c)
Leaf width
(cm) - width of the third leaf from the top at its widest part was measured.
d) Single
leaf area (cm2) - was calculated using the formula KxLxW, where K is
the “adjustment factor” 0.747, L is length and W is the width (Stickler and
Wearden, 1961).
e) Plant
height (cm) – measured from the base of the stalk at ground level to the tip of
the head.
f)
Head
length (cm) - measured from the base of the panicle to the tip of the panicle.
g) Head width (cm) - the diameter of the head at its widest part
h) Head
weight (g) - the mean value of 5 plants head weight was measured.
a.
Days to emergence: The number of days from
sowing to 50% emergence of the plants.
b.
Days to flowering: The number of days from
planting to when 50% of the plants in plot reached 50% flowering.
c.
Days to maturity: The number of days from
planting to the date where 95% of the plant matured on which seeds on the lower
part of panicle formed black layer.
d.
Head shape: Scored on a 1-3 scale.
e.
Head compactness: scored on a 1-12 scale.
f.
Inflorescence exertion: This was scored on a
1-4 scale.
g.
Grain covering with glumes: Scored by visual
observations of the grain on the whole plants from the plot
h.
Grain color: It was scored based on visual
observations as white, brown and red.
i.
Grain yield (g): Weight of dried grain
harvested from the two rows of the plot
Before analysis of variation, the overall data set
was divided into two groups. One group contained quantitative data and the
other group contained qualitative data, which were coded by numbers(Table
2). For quantitative data, mean value was used for all characters but for the
coded data with variation within one accession, a decision was made to use
dominant character and only one code was used per accession. After verification
of the data subset, frequencies of occurrence of each qualitative character
were calculated by hand through percentage comparison method. Eleven recorded quantitative data were
subjected to statistical analysis using NCSS, 2004, SAS statistical computer
software with version 9.2 (SAS institute, 2008) and GenRes statistical computer
software with version 3.11 (Pascal institute, 1994). Statistical measure of
variability, such as genotypic and phenotypic coefficient of variation, broad
sense heritability, genetic advance and genetic advance as percent of the mean,
phenotypic correlation coefficient, path analysis and cluster analysis were
performed using the above appropriate statistical computer software programs.
Analysis of variance (ANOVA) was performed (Table 3) for each measured
quantitative trait in
RCBD ANOVA
(Gomez and Gomez, 1984; Rangswamy, 1995) in order to
compare the relative importance of main model terms: replication(r), genotype
(G) and error (e). Analysis
of variance (ANOVA) was performed on eleven quantitative traits to explore the
level of variation among the twenty-five populations of sorghum using NCSS, 2004 computer statistical program.
The RCBD
ANOVA was computed using the mathematical model:
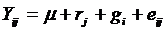
Where:  = the observation of the ith
genotype in the jth replication,
= the observation of the ith
genotype in the jth replication,  =the
effect of the jth replication and
=the
effect of the jth replication and  = the effect of
the ith genotype, eij=
experimental error effect.
= the effect of
the ith genotype, eij=
experimental error effect.
The genetic parameters were estimated using
SAS statistical computer software with version 9.2 (SAS institute, 2008).
The phenotypic and genotypic variances were
estimated from the expected mean squares using the random model where the
expected mean squares considered. The mean squares equated to their
expectations for the estimation of variance components. Genotypic variance and phenotypic
variance
and phenotypic
variance were estimated
according to Falconer (1981) as:
were estimated
according to Falconer (1981) as:  =
= , Where,
, Where, 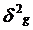 is genotypic
variance,
is genotypic
variance,  is genotypic mean square,
is genotypic mean square,  = error mean squares, r is the number of replications.
= error mean squares, r is the number of replications.
Environmental variance on genotype mean basis
 =
= , and Phenotypic variance
, and Phenotypic variance  =
=  +
+  =
= , Where,
, Where, 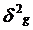 is phenotypic
variance. Variability was estimated using range, standard error, phenotypic and
genotypic variances, and phenotypic and genotypic coefficient of variation. The
phenotypic and genotypic coefficients of variation calculated according to the
Burton (1952) method as:
is phenotypic
variance. Variability was estimated using range, standard error, phenotypic and
genotypic variances, and phenotypic and genotypic coefficient of variation. The
phenotypic and genotypic coefficients of variation calculated according to the
Burton (1952) method as:
PCV = 
 (
(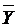 …
…
 , Where
, Where 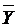 is mean value
of trait Y.
is mean value
of trait Y.
 =
= 
 (
(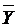 …
…

B.
Estimation of heritability in broad sense (H)
Heritability in broad sense (H) for all
characters was computed using the formula given by Falconer (1981). Broad sense heritability (H) expressed as a
percentage of the ratio of the genotypic variance  to the phenotypic variance
to the phenotypic variance  and was estimated on genotype mean base as described
by Allard (1960).
and was estimated on genotype mean base as described
by Allard (1960).
Heritability , where:
, where:  is heritability
in broad sense,
is heritability
in broad sense,  is Genotypic
is Genotypic
Variance, and  is phenotypic
variance.
is phenotypic
variance.
Genotypic advance in absolute unit  and as percent of the mean
and as percent of the mean , assumed selection of superior 5% of the genotypes
was estimated in accordance with the methods illustrated by Johnson et al. (1955) as:
, assumed selection of superior 5% of the genotypes
was estimated in accordance with the methods illustrated by Johnson et al. (1955) as:
 =K
=K
 , where: K is
the standardized selection differential of 5% selection intensity. (K = 2.063),
, where: K is
the standardized selection differential of 5% selection intensity. (K = 2.063),
 is phenotypic
standard deviation on mean basis, H is heritability in broad sense. Genetic
advance as percent of the mean was calculated to compare the extent of
predicted advance of different traits under selection, using the formula:
is phenotypic
standard deviation on mean basis, H is heritability in broad sense. Genetic
advance as percent of the mean was calculated to compare the extent of
predicted advance of different traits under selection, using the formula:  Where:
Where:  is mean of the
population where selection employed.
is mean of the
population where selection employed.
Correlation and path
analysis between days to 50% flowering, days to maturity, number of leaves per
plant, single leaf area, leaf length,
leaf width, plant height, head
length, head width, head weight and grain yield of 25 sorghum accessions were
studied using SAS computer software with version
9.2 and GenRes computer software with
version 3.11 computer software programs, respectively.
Covariance analysis followed the same fashion
as that of analysis of variance, and the mean cross products were equated with
their expectations to solve the covariance component as:
Genotypic covariance (δ2gxy)
= 
Phenotypic covariance
Phenotypic correlation, the observable
correlation between two variables, which includes both genotypic and
environmental components between two variables were estimated using the formula
suggested by Miller et al. (1958) as
indicated on the next page.


Where, rpxy
is phenotypic correlation coefficient and rgxy is genotypic
correlation coefficient between character x and y; δ2pxy and
δ2gxy are phenotypic covariance and genotypic covariance
between character x and y respectively.
Phenotypic (δ2pxy)
and genotypic (δ2pxy) covariance was computed from
the table of covariance analysis in a manner similar to that of the analysis of
variance (Table 4).
Path coefficient analysis was analyzed using
the formula suggested by Dewey and Lu (1959) to assess the direct and indirect
effect of yield components on seed yield based on the following relationship
with GenRes statistical computer software with version 3.11(Pascal institute,
1994).
 , Where:
, Where:  is mutual association between the independent
character (
is mutual association between the independent
character ( ) and dependent character, grain yield (
) and dependent character, grain yield (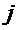 ) measured by the correlation coefficient,
) measured by the correlation coefficient,  is component of
direct effects of the independent character (
is component of
direct effects of the independent character ( ) on the dependent variable (
) on the dependent variable (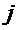 ); as measured by the path coefficients,
); as measured by the path coefficients,  is the summation of components of indirect effect of a
given independent trait (
is the summation of components of indirect effect of a
given independent trait ( ) on the dependent variable (
) on the dependent variable (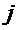 ) via all other independent traits (k). The
contribution of the remaining unknown factor measured as residual factor (R2) was calculated using the
formula (Dewey and Lu, 1959) as:
) via all other independent traits (k). The
contribution of the remaining unknown factor measured as residual factor (R2) was calculated using the
formula (Dewey and Lu, 1959) as:
 ,
,
Where , U=the residual (unexplained variation of the
dependent variable that is not accounted for by path coefficients). In this
study, grain yield was considered as the dependent trait and all other ten
quantitative characters were taken as independent traits.
, U=the residual (unexplained variation of the
dependent variable that is not accounted for by path coefficients). In this
study, grain yield was considered as the dependent trait and all other ten
quantitative characters were taken as independent traits.
Mahalanobis’s generalized distance (D2)
statistics was used for assessing the divergence between genotypes based on the
number of traits that were measured using NCSS, 2004 computer statistical
program. The generalized distance between any two populations defined as:
 = (
= ( –
–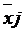 ) S-1 (
) S-1 ( –
–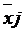 ),
),
Where,  = the distance between any two groups
= the distance between any two groups 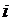 and
and ,
,  and
and 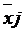 = are the vector mean of the traits for the ith and jth
groups respectively, and S-1 is the inverse of the pooled covariance
matrix.
= are the vector mean of the traits for the ith and jth
groups respectively, and S-1 is the inverse of the pooled covariance
matrix.
Morphological variation was recorded among the 25
germplasm of sorghum bicolor
collected from different areas of north western Benishangul Gumuz Regional
State, Ethiopia. The 5 qualitative and 11 quantitative characters varied among
the 25 sorghum germplasm.
Variability was observed in five
traits including head shape and compactness, inflorescence
exertion, grain covering with glumes and
grain colour.
The landraces were characterised
by straight head (87.5%), semi-curved head (4.2%) and curved head (8.3%). The
variety that was included as a standard check was of the straight-headed type.
Some sorghum landraces, which were collected from North
Shewa and South Wollo, had Straight head (Abdi et al., 2002). Variations in
head shape and compactness have also been reported from landraces of South Wollo
(Solomon and Lemlem, 2003). Durrishahwar et al. (2012) also observed variation
for head shape in Pakistan.
The landraces also showed variation on head compactness as loosely
headed (58.3%), semi-compact headed (20.8%) and compact headed (20.9 %). The
reason for the observed high percentage of loosely headed landraces than the
compact headed and semi compact headed landraces seems that there was targeted
selection of materials for grain mould resistance in the areas where the
accessions were collected among the local farmers. Stemler et al. (1977), Rao and
Mengesha (1981) and Thakur
et al. (2008) explained that the
loosely headed sorghums are more resistant to grain mould (a fungal disease)
than compact headed sorghums. Because the loosely headed sorghums have an
adaptive trait (open panicle) which facilitates quick drying of the panicle in
areas of high rainfall and humidity, thereby minimizing grain weathering due to
fungal diseases such as grain mould. Amsalu and Endashaw
(1998) also reported a variation on head compactness.
Contradictory to
this study, Abdi et al. (2002)
reported 33% of 34 accessions were compact headed which are dominant in North Shewa and South Welo. Amsalu and Endashaw (1998) reported loosely
headed types with dropping branches occur abundantly in relatively cool and wet
regions of Ethiopia like Wollega, Illubabur, Shewa and Sidamo where
susceptibility to grain mould is high due to the increase in humidity.
The inflorescence exertion,
measured as the amount of exposed peduncle from the flag leaf to the base of
the panicle was recorded to be slightly exerted (64%), exerted (20%), and well
exerted with long peduncle (8%) and peduncle curved (8%). Habindavyi (2009) reported 48% well exerted, 36% exerted and 17 % less
exerted landraces from 50 sorghum landraces of Burundi. A case study by Solomon
and Lemlem (2003) indicates that there is a greater variability of sorghum
landraces of north-eastern Ethiopia on their inflorescence exertion. Abdi et al. (2002) also reported variability
among 34 sorghum accessions in their inflorescence exertion (59% of the
accessions with well exerted inflorescence).
The genotypes produced white
(48%), red (32%) and brown (20%) coloured seeds. This seems that the result of
conscious artificial selection chosen genotypes with white colour and
discriminates genotypes with brown and red colour seeds. In the same way Durrishahwar et al. (2012) reported white seeded
sorghum as the most frequent landraces in Pakistan. Habindavyi (2009) also reported brown sorghum grains as the second
dominant (25%) grain colour when dealing on morphological characterization of
52 sorghum landraces in Burundi. The author reported red grains as the most
dominant (52%) and white coloured grains as the least dominant (23%). Amsalu
and Endashaw (1998) discussed that white and brown seeds were the most frequent
seed types of sorghum collected from different areas of Ethiopia and Eritrea. However,
they concluded that red sorghum seeds are more
frequent than brown seeds of sorghum. Solomon and Lemlem (2003) also reported variability in grain colour of
sorghum accessions in their case study folk classification of sorghum in
north-eastern Ethiopia. Dogget (1982) stated white colour
seeded sorghums are known for their food quality despite their vulnerability to
grain mould. The white grain sorghum lacks polyphenolic compounds that serve to
protect the sorghum grain from pre-harvest germination in humid regions in
intermediate and highland areas, while red and brown colour, seeded types are
rich in polyphenolic compounds (Ashante, 1995).
The observed frequencies of the different colour categories in this
study could be associated with the utilization and disease tolerance aspects of
the crop. Sorghum is the staple food in the whole collection areas where white
colour grain types make better quality ‘injera’ and porridge than the other two
grain types. The brown and red colour grain types are mainly used for making a
local homemade alcoholic drink called ‘Tella’. Besides, the white seeded
sorghums have better market price than the other two sorghum types and that is
why these white seeded sorghums have larger land covers than brown and red
seeded types of sorghum.
Sixty percent of the landraces
had 25% covered grains, 36% of the landraces had grains that are half covered,
and 4% of the landraces had grains that are fully covered with glumes. This
seems to have favoured grains either half covered or 25% covered with the
glumes. This seems to be an adaptive feature that facilitates quick drying that
minimizes grain mould. Habindavyi (2009) reported the most dominant 25% covered
grains (47%), second dominant (35%) half covered grains and the least dominant (2%)
sorghums with fully covered grains. Elangovan et al. (2007) reported a glumes covering
variation among sorghum accession. Thakur
et al. (2008) also reported a
variation of sorghum landraces in their grain covering and concluded that
greater glumes coverage of grains appeared to be more closely associated with
grain mould resistance than other traits.
The significant mean square values obtained from
the analysis of variance suggests that differences existed among the sorghum
germplasm for most characters, indicating that they are highly variable (Table 6).
The results indicated that there
is a significant difference (P<0.05) among genotypes for the traits such as
days to 50% flowering, days to maturity, number of leaves per plant, plant
height, head weight and grain yield. There is also a significant difference
(p<0.01) for the traits of leaf length, single leaf area, and head length.
But the genotypes did not significantly vary for the traits of head width and
leaf width. The significant difference obtained indicates the presence of
genetic variability for those traits with a significant mean square value
(Table 6). A higher variation for a character in the breeding
materials correlates with a greater ability for its improvement through
selection (Mahdieh et al., 2012). The CV is useful
when comparing the experimental variation differences in experiments that have
variables measured in different units and large coefficients of variation
(>30%) are often associated with increased experimental variability (Taylor et al., 1999). Therefore, grain yield (CV=19.08) has the greater ability to be improved through
selection and days to 50% flowering (C.V =2.65) correlated with a least ability
for its improvement among the 11 quantitative traits. Non-significant mean
square values observed for some characters showed that the genotypes are
genetically similar concerning these characters. Selecting for these characters
will therefore show no impact on genetic improvement (Bello et al., 2007). Therefore, leaf width and
head width will not have impact on genetic improvement of the sorghum
accessions.
Previous studies on collection of sorghum landraces indicate significant
variation for many of the traits like head length and head width, days to 95%
maturity (Habindavyi, 2009). Negash
(2003) also reported that days to 95% maturity, leaf width, leaf length, leaf
area, plant height and grain yield have a significant difference between
sorghum landraces collection from Western region of Ethiopia. Doggett
(1988) and House (1985) showed the presence of substantial amount of
variability among sorghum genotypes for different agronomic traits like field
emergence, days to maturity, and time to flowering. In contrast to the study of Negash (2003) variability is not obtained
in days to emergence in the present study. Wasihun (2007) obtained a result
similar to this finding except for days to 50% flowering, which has a
significant difference in this study.
A wide range of values in
quantitative traits has been observed (Table 7). The sorghum germplasms have a
broad range of variability in grain yield per plot (4538 g to 2174 g/plot or 60
to 28.9 q/ha). The studied genotypes also showed range of variability in their
days to 50% flowering (41-128 days), days to 95% maturity (130-170 days), leaf
length (71.8-112.8 cm), number of leaves (458-895) and leaf width (8-17 cm) and
in single leaf area (7.07-9.6 cm2).
According to Amsalu (2000), mature sorghum leaves may reach a width of
6.5 to 13.46 cm at the widest point and the number of leaves on the main stem
may vary from 6.96 to 20.63. In a related study by Geremew (1993), leaf length
was reported to a range of 45.22–126.37 cm. The variability in plant height
among genotypes was high and ranged between 423-203 cm. Variability in plant
height of Ethiopian sorghum ranged from 72 to 615 cm has been reported by
Berhane and Yilma (1978). Variation in head length, head weight and head width
was also observed in this study with a range of variability from 43.2-19.00 cm,
120.8-47.00 g and 12-5 cm respectively. Almost similar result was obtained by
Melakhail and Rao (1982) and Wasihun (2007). Therefore, the existence of wider
morphological diversity among the sorghum landrace collections implies the
potential to improve the crop and the need to conserve these resources.
Mean grain yield of the accessions was 2988.63 g/plot that is 39.84 q/ha
with the variation ranging 60 q/ha to 29 q/ha. Genotype 021PW-2010 was the
highest yielder landrace (48.9 q/ha) which is almost less by 11 quintals when
compared to the check variety (Emohay having a potential to give 60 q/ha).
Eleven landraces gave between 40.2 - 40 q/ha grain yields. These landraces were 044pw-2010, 035pw-2010, 001pw-2010, 021pw-2010,
050pw-2010, 056pw-2010, 049pw-2010, 055-2010, 041pw-2010 and 023pw-2010. The
mean values of the quantitative characteristics studied are shown in Table 5.
The presence of genotypes with good yield performance is an indication for the
possibility of developing high yielding varieties from the available materials
for Pawe and similar areas.
Variability parameters for different
morpho-physiological characters were assessed to determine patterns of
genetic variation among the genotypes. Variability present within the sorghum germplasm estimated from the
range of values for phenotypic and genotypic coefficients of variation,
heritability and genetic advance of each character. Generally, the GCV
are lower in magnitude than the PCV. Similar results were obtained by Bello et al. (2007) while
studying genetic variability in
cultivated sorghum. Abdi et al.
(2002) and Addisu (2011) also reported a variability result on the quantitative
characteristics of sorghum germplasm.
A.
Phenotypic and genotypic
variation among the genotypes
Across the 11 characters, the genotypic and
phenotypic coefficient of variation (GCV & PCV) ranged from 5.9% to 6.1%
for days to 95% maturity and 15.8%
to 16.2% for plant height, respectively, as indicated on table 7. Negash (2003) and Wasihun (2007) obtained the lowest PCV and GCV for
days to 95% maturity. Addisu (2011) also reported the lowest genotypic and phenotypic coefficients of variation for 95% days to maturity
(8.22% and 8.75% respectively) but in contrast, he recorded the highest GCV and
PCV for days to 50%flowering. Godbharle et
al. (2010) reported high genotypic and phenotypic coefficient of variation
for pant height, head width and head length, and low GCV and PCV for days to
flowering (5.86% and 6.85% respectively). If The PCV was higher than the GCV
for the traits, the traits will be highly influenced by environment and the
reverse is true (Godbharle et al.,
2010). Therefore, in the present study
the high ratios of the phenotypic variance to genotypic variance for days to
flowering, days to maturity, leaf length, leaf width, single leaf area, number
of leaves per plant, plant height, head length, head width, head weight and
grain yield indicated that the traits will be highly
influenced by environment.
B.
Heritability
estimates in broad sense
Although the genotypic
coefficient of variation revealed the extent of genetic variability present in
the landraces for various traits, it does not provide full scope to assess the
heritable variation. Heritable variation is useful for permanent genetic
improvement (Jalal et al., 2011). The
most important function of heritability in the genetic study of quantitative
characters is its predictive role to indicate heritability of the phenotypic
value as a guide to breeding value (Falconer and Mackay, 1961).
High heritability estimates for single leaf
area (99.62%), days to 50% flowering (97.8%), plant height (95.7%), number of
leaves per plant (94.3%), leaf length (93.9%), and days to 95%maturity (93.5%) and leaf width (70%) were obtained
(Table 7), indicating a high response to selection. In a similar fashion, a
high heritability for head length (96%), days to 50% flowering (95%), and days to 95% maturity (99%) was reported by Bello et al. (2007). Mahajan et al. (2011) also reported a high
heritability in plant height (92.12%), head width (92.97%) and days to 50%
maturity (89.57%). A low heritability for head width (59.7%), head weight
(45.1%) and grain yield (51.6%) recorded in the present study. Therefore, the masking effect of the
environment is high on these traits and selection for the traits will not be
easy for the plant breeder. Deepalakshmi and Ganesamurthy (2007) reported a
high heritability in days to 50% flowering (94.6%), 95% days to maturity (90.80%), and in head weight (96.90%). Negash
(2003) reported high heritability for days to 50% flowering (77%), plant height (78.50%) and head length
(84.80%). Wasihun (2007) also reported high heritability for days to 50% flowering (97%), days to 95% maturity (99%), leaf length (75%), single leaf area
(75%), and plant height (94%) and grain yield (94%). A lowest heritability in
head weight (45.10%) and a highest heritability in single leaf area (99.62%)
were observed. Bellow et al. (2007)
reported a lowest heritability in grain yield (10%) but Deepalakshmi and
Ganesamurthy (2007) reported the highest heritability in grain yield (98.7%).
In general, heritability was high for most quantitative characters
studied suggesting that selection for those traits would be effective.
Nevertheless, it should be noted that this is broad sense heritability and
hence it is not absolute indicator of efficiency (Singh, 1999). According to
Singh (1999), if heritability of a character is very high, say 80% or more,
selection for that character is easy, but the masking effect of the environment
is high on traits with low heritability (less than 40%). Therefore, selection
for single leaf area (99.62%), days to 50% flowering (97.8%), plant height
(95.7%), number of leaves per plant (94.3%), leaf length (93.9%) and 95% days to maturity (93.5%) will be easy for plant breeders.
C.
Estimates of genetic advance
In this study estimate of genetic
advance (GA) ranged from 1.39 cm for leaf width to 9.65q/ha for grain yield
(Table 7). That is the resultant population obtained after crossing the best 5%
of the materials will produce a new population whose leaf width is increased by
1.39 cm and whose yield is better than the older population by 9.65 q/ha. Low
predicted response to selection was observed for leaf length (16.83 cm), head
weight (13.6 cm), head length (9.64 cm), days to 50% flowering (5.78 days),
number of leaves per plant (4.20), and days to 95% maturity (2.06), head width
(1.77 cm) and for leaf width (1.39 cm). A higher value of GA was observed for
single leaf area (220.96 cm2), plant height (116.35 cm) and grain
yield (9.65 q/ha).
Wasihun (2007) reported high values of GA for grain yield (880.33 g) and
single leaf area (152.25 cm2). Negash (2003) also reported high
values of GA for plant height (82.2 cm) and single leaf area (91.3 cm2).
He also reported GA less than 10 for number of leaves per plant, head length,
head width and head weight. Godbharle et
al. (2000) reported a high value of GA in kharif sorghum. Deepalakshmi and Ganesamurthy (2007) also reported high GA for plant height (31.45 cm) and head length
(29.12 cm). The genotypic coefficient of variation along with heritability
estimate provides reliable estimates of the amount of genetic advance expected
through phenotypic selection (Burton, 1952).
GAM was
moderate for all characters except days to 50%
flowering, which recorded its low estimate (5.1%).
Maximum GAM recorded for plant height (33%) followed by head length (32.9%) and
number of leaves per plant (30%). Similarly, Wasihun (2007) reported low GAM in
days to 50% flowering (0.15%) among the sorghum collections in western
Ethiopia. He reported low to high GAM for days to 95% maturity (0.11%), leaf
width (4.12%), leaf length (0.46%), single leaf area (24.94%), number of leaves
per plant (3.31%), plant height (0.2%), head width (26.63%), head length
(2.23%), head weight (42.17%), and Grain yield (58.52%). Deepalakshmi and
Ganesamurthy (2007) also reported high GAM for
plant height, days to 50% flowering, number of leaves per plant, leaf length and head weight.
The estimate of GA helps in understanding the type of gene action
involved in expressing various polygenic characters when considered jointly
with heritability. High values of GA are indicative of additive gene action
where as low values are indicative of non-additive gene action (Singh and
Narayanan, 1993). Thus, days to 50% flowering estimated high heritability
(97.8%) and low genetic advance as percent of the mean (5.78%) indicating that
this character is affected by environment and is controlled by non-additive
gene action and it will have poor response for selection (Godbharle et al., 2010). Days to 95% maturity (H=93.5%;
GAM=12.7%), leaf width (H=70%; GAM=19.7%), leaf length (H=93.9%; GAM =16.9%),
head length (H=92.8; GAM=32.9%), head width (H=45.1%; GAM=16.6%), and grain
yield (H=51.6%; GAM=24.33%), which expressed high heritability with moderate
GAM, appeared less affected by environmental fluctuations and governed by both
additive and non-additive gene action, suggesting the possibility of improving
these characters through simple selection methods. Deepalakshmi and Ganesamurthy (2007) also confirmed this statement. High heritability coupled with moderate
GAM for head length reported by Godbharle et
al. (2010).
The association of all morphological traits
was estimated by genotypic and phenotypic correlation coefficients (Table 8). The higher
magnitude of genotypic correlation
coefficients (rg) than phenotypic
correlation coefficients (rp) recorded in a correlation of days to
50% flowering with leaf width, head length and head weight, days to 95%
maturity with head length, head weight and grain yield, leaf length with leaf
width, head length, head weight, number
of leaves per plant with leaf width, and head weight, leaf width with plant
height, head weight, and head length, single leaf area with head length, and
grain yield with head length, head width, and head weight. Godbharle et al. (2010) stated that traits with
higher genotypic correlation have inherent association. Therefore, the above
quantitative traits in this study with higher genotypic but lower phenotypic
correlation coefficient have inherent association.
There was
positive and significant genotypic correlation of grain yield with days to 95%
maturity (rg = 0.69**), head length (rg =0.74*), head
width (rg = 0.33**) and head weight (rg = 0.63**)
indicating that increase in grain yield is because of increase in one or more
of the above characters. Similar results were reported by Bohra et al. (1986) for head length,
Deepalakshmi and Ganesamurthy (2007) for number of leaves per plant and head
weight, and Ezeaku and Mohamed (2006) for head weight. Grain yield is
significantly and negatively correlated with single leaf area (rp =
-0.25*) and number of leaves per plant (rp = -0.32**) at phenotypic
level (Table 8). Geremew (1993), Negash
(2003) and Wasihun (2007) reported that grain yield is positively and
significantly correlated with head weight. Deepalakshmi and Ganesamurthy (2007) reported that days to 90% maturity and head weight are positively
and significantly correlated with grain yield and a negative significant
correlation between number of leaves per plant and grain yield. Elangovan et
al. (2007) reported a positive and significant correlation of head width
with grain yield at genotypic level. In
agreement with this study, Ezeaku and Mohamed (2006) also reported a positive
correlation between plant height and head length; head length and head weight;
head length and grain yield but in contrast, a negative correlation between
plant height and head weight; plant height and grain yield; and head weight and
grain yield.
3.4.
Path Coefficient Analysis
The
estimates of correlations alone may be often misleading due to mutual
cancellation of component traits. The path coefficient
analysis initially suggested by Wright (1921) and described by Dewey and Lu
(1959) allows partitioning of correlation coefficients into direct and indirect
contributions (effects) of various traits towards the dependent variable and
thus helps in assessing the cause-effect relationship as well as effective
selection. Hence, this study aimed to analyse and determine the traits having
greater inter-relationship with grain yield utilizing the correlation and path
analysis.
The
results of path analysis (Table 9) revealed that days to 50% flowering (0.38),
days to 95% maturity (0.45), leaf length (0.14) and head length (0.28) showed
positive direct effect with grain yield. Leaf width (-0.58), single leaf area
per plant (-0.18), number of leaves per plant (-0.17), plant height (-0.76),
head width (-0.15) and head weight (-0.19) showed a negative direct effect on
grain yield per plot. This shows that direct selection of days to 50%
flowering, days to 95% maturity, leaf length and head length will be effective
in improving sorghum grain yield but increasing leaf width, single leaf area
per plant, number of leaves per plant, plant height, head width and head weight
through selection may not necessarily lead to proportionate increase in grain
yield. Similarly, Mahajan et al.
(2011) reported positive direct effect of head length (0.4036) on grain yield
and contradicted to this finding a negative direct effect on days to 50% flowering
and days to 95% maturity and a positive direct effect on head width (0.8432)
and plant height (0.4329).
Deepalakshmi and Ganesamurthy (2007) reported that days to 50 %
flowering (0.129), days to 95% maturity (0.491), head length (0.438), leaf length (0.070) have a
positive direct effect on sorghum grain yield. Moreover, plant height (-0.149)
has a negative direct effect on grain yield. Contradictory to this finding he
reported a positive direct effect of number of leaves per plant (0.820) and
head weight (0.854) on grain yield. Days to maturity (0.45) showed the highest positive direct effect while
leaf length (0.14) showed the least positive direct effect and plant height
(-0.79) showed the highest negative direct effect while head width (-0.15)
showed the least negative direct effect on grain yield per plot. Ezeaku and
Mohamed (2006) reported a highest positive direct effect of head weight (0.97)
and the lowest positive direct effect of head length (0.03) on grain yield.
According
to the study of Mahajan et al. (2011)
days to 50% flowering has the highest negative direct effect
(-0.1570) and days to 95% maturity have the least negative direct effect (-0.0935) on grain yield. Mahajan et al. (2011) stated that head
width, head length and plant height, which have positive direct effect on grain
yield, had greater importance in improving grain yield. Thus, it revealed from the present study
that the traits like days to 50% flowering, days to 95% maturity, leaf length
and head length had greater importance. Hence, due consideration should be
given to these characters while planning a breeding strategy for increased
grain yield in sorghum. The residual effect
(0.63) indicates that characters, which are included in the path analysis,
explained almost 99.36% of the total variation in seed yield.
Eleven quantitative
characters were used as input for cluster analysis. Hierarchical method of
cluster analysis was performed using NCSS, 2004 computer software program based
on a dissimilarity matrix and a considerable variability observed between the
clusters. However, the landraces have been collected in different areas, some
are similar and sharing cluster (grouped in a cluster) and some others
originally collected from similar area are placed in different clusters. Thus,
even if the landraces are originally collected from different area, they are
sharing some morphological characteristics. The 25 sorghum
germplasm grouped in 5 clusters (Fig. 1). The number of landraces per cluster
ranged from one in cluster I to eight in cluster III (Table 10).
Cluster I consisted of only the local check (Emohay) which is
characterized by high yield, short leaves, early maturity and shorter height
which makes it easy for harvesting. Cluster II contains 4 genotypes having
early flowering and maturity date. The shortest genotype is included in this
cluster and this makes the genotype most suitable for harvesting but it is
characterized on average, by lower grain yield. But among
the landraces, the highest grain yielder germplasm (021pw-2010 which gave 3565
g/plot or 48.9q/ha) was found in this cluster. Cluster III consisted of eight genotypes that have late maturity date
(162.26 days) relative to the standard check and cluster II and IV member sorghum
genotypes, intermediate plant height and higher grain yield among the tested
sorghum landraces (3037.8 g/plot or 41.67 q/ha). They have longer leaves (102.97 cm).
Cluster IV and V are composed of intermediately flowering (118.16-118.71 days) and maturing (162.21-162.93 days) accessions. Both clusters are composed of six genotypes, which
contain high yielder genotypes. Cluster V consisted
of taller accessions (394.95 cm average plant height), which will make them
difficult for harvesting. The tallest landrace (412.3 cm) 026pw-2010 was also
found in this cluster. Diversity for sorghum accessions was also reported
earlier (Abdi et al., 2002, Wasihun, 2007, Abe, 2010 and Durrishahwar et al., 2012).
For all the clusters, the distance between clusters
centred (D2) detected (11). All the clusters were different from one
another indicating that the sorghum landraces under the study varied
morphologically. The cluster mean
values of the 11 quantitative characters were computed to compare the clustered
accessions of sorghum as indicated on table 12.
Table 1. List of sorghum landraces and the standard check included
in the study
|
No
|
Accession
No
|
Local
Name
|
Collection
area
|
Altitude
|
Seed
Color
|
|
1
|
033pw-2010
|
Bobea
|
Village 49
|
894
|
Red
|
|
2
|
037pw-2010
|
Bobea
|
Village 1
|
1044
|
Brown
|
|
3
|
035pw-2010
|
Bobea
|
Village 1
|
1044
|
White
|
|
4
|
043pw-2010
|
Bobea
|
G/beless
|
980
|
Brown
|
|
5
|
044pw-2010
|
Bobea
|
Village 1
|
1044
|
White
|
|
6
|
045pw-2010
|
Bobea
|
Village 1
|
1044
|
Red
|
|
7
|
026pw-2010
|
Auto
|
Icide
|
915
|
Brown
|
|
8
|
030pw-2010
|
Auto
|
Icide
|
915
|
Brown
|
|
9
|
047pw-2010
|
Bobea
|
Baruda
|
-
|
red
|
|
10
|
001pw-2010
|
Mehareb
|
Almehal
|
680
|
White
|
|
11
|
003pw-2010
|
Tirequash
|
Almehal
|
685
|
White
|
|
12
|
012pw-2010
|
Gergesa
|
Banguze
|
808
|
White
|
|
13
|
020pw-2010
|
Drisisa
|
Icica
|
890
|
White
|
|
14
|
021pw-2010
|
Jarsisa
|
Icide
|
890
|
White
|
|
15
|
050pw-2010
|
Bobea
|
Dibate
|
-
|
White
|
|
16
|
056pw-2010
|
Bobea
|
Dibate
|
-
|
White
|
|
17
|
005pw-2010
|
Bobea
|
Village 23
|
-
|
Red
|
|
18
|
049pw-2010
|
Bobea
|
Baruda
|
-
|
Red
|
|
19
|
055pw-2010
|
Bobea
|
Zeghe
|
-
|
White
|
|
20
|
031pw-2010
|
Bobea
|
Village 1
|
1044
|
White
|
|
21
|
032pw-2010
|
Bobea
|
Village 49
|
894
|
White
|
|
22
|
027pw-2010
|
Auto
|
Icide
|
915
|
Red
|
|
23
|
041pw-2010
|
Bobea
|
Village 1
|
1044
|
White
|
|
24
|
042pw-2010
|
Bobea
|
Village 1
|
1044
|
Red
|
|
25
|
023pw-2010
|
|
Dechagree
|
862
|
Brown
|
|
26
|
Emohay
|
Check
|
-
|
-
|
Brown
|
Table 2. Qualitative characters scored following
the sorghum descriptor lists (IPGRI/ICRISAT, 1993)
|
|
Characters/Codes/
Description
|
|
1.
|
Grain
color. Codes: 1. White; 2. Brown; 3. Red
|
|
2.
|
Head
shape. Codes: 1. straight; 2.
Curved; 3. Semi curved
|
|
3.
|
Head compactness.
Codes: 3. Loose: 7. Semi compact 9. Compact
|
|
4.
|
Inflorescence
exertion. Codes: 1. Slightly exerted; 2. Exerted; 3. Well exerted;
4.
Peduncle curved
|
|
5.
|
Grain
covering with glumes. Codes: 1. 25% grain covered: 3. 50% grain covered: 5.
75% grain covered 7. Grain fully covered: and 9. glumes longer than grain
|
Table 3. Analysis of variance for 11 traits for the 25 sorghum germplasm
|
Source of variation
|
df
|
Mean square
|
Expected mean
|
|
Replications
Genotypes
Error
|
(r-1)
(g-1)
(r-1) (g-1)
|
MSr
MSg
MSe
|
δ2e-gδ2r
δ2e-rδ2g
δ2e
|
|
Total
|
rg-1
|
|
|
df = degrees of freedom, r = number of
replication, g = number of genotypes, MSr = mean
square of replications, MSg = mean square of genotypes, MSe
= mean square of error.
Table 4. Analysis of covariance
|
Source of variation
|
df
|
Mean square
|
Expected mean
|
|
Replication
|
r-1
|
MSCPrxy
|
|
|
Genotype
|
g-1
|
MSCPgxy
|
δ2exy+rδ2gxy
|
|
Error
|
(r-1) (g-1)
|
MSCPexy
|
δ2exy
|
Where: df-degrees of freedom, r-number of
replications, g-number of genotypes, MSCPrxy-mean sum of cross
products of replication for traits x and y, MSCPgxy-sum of cross
products of genotype for traits x and y, MSCPexy-sum of cross
products of environment for traits x and y, δ2exy-environmental
covariance between traits x and y, δ2gxy-genotype
covariance of traits x and y.
Table 5.
Genotype Mean summary of 11 quantitative characters and values obtained for
five qualitative characters of 25 sorghum genotypes grown in 2011/2012 rainy
season in Pawe district
No. Accessions DTE DTF DTM LFW LFL SLA NOL PH HL HW HWt GRY IEX HS HC GLC GRC
1 033pw-2010 7 120 165 7.6 99.5 754.1 15 385 27.7 9.3 93 3329.3 1 3 3 3 3
2 037pw-2010 7 112 160 7.4 104.5 758.1 14 379.7 33.6 10.5 91.1 2174 1 3 3 1 2
3 035pw-2010 7 94 139.7 6.1 86.7 535.8 11 244.7 28.6 7.2 88.9 3017 1 3 3 3 1
4 043pw-2010 7 117.3 161.7 7.1 100.4 703.5 14 346.7 28.5 9.3 76.4 2641.7 1 3 3 1 2
5 044pw-2010 7 120.3 161.7 7.4 105.5 781.7 15 309.3 24.1 9.3 73.1 3152.3 4 1 9 1 1
6 045pw-2010 7 122 160 5.6 101.7 593.2 15 372.6 27.4 7.1 80.8 2643.3 1 3 7 3 3
7 026pw-2010 7 117.7 163 8.2 101.5 741.8 15 412.3 32.2 9.6 86.7 2462.6 3 3 3 3 2
8 030pw-2010 7 119.3 161.3 8.1 108.1 870.3 15 404.3 30.3 7.9 79.3 2302.3 2 3 7 3 2
9 047pw-2010 7 122.7 161.3 7.9 105.9 834.4 14 354 25.2 7.5 70 2847 1 3 9 1 3
10 001pw-2010 7 91.3 138.3 6.7 89.3 607.1 11 245 24.6 6 66.9 3375.7 1 3 3 1 1
11 003pw-2010 7 90.3 137.3 7 90.3 641.9 10 236.7 30.3 6.7 89.2 2829.7 2 3 3 1 1
12 012pw-2010 7 96.7 140.3 8.4 87.9 740.7 12 284 26.8 8.3 64.5 2596 1 3 7 1 1
13 020pw-2010 7 114.7 161.7 7.7 98.4 759.7 16 365.7 28.3 7.8 80.9 2549.7 1 3 9 3 1
14 021pw-2010 7 115 163.3 6.5 107.3 706.2 14 359.3 30.2 8.1 85.1 3565 2 3 9 1 1
15 050pw-2010 7 122 165 7.4 101.1 766.1 15 386.7 40.5 9.3 98.4 3347.7 1 3 7 1 1
16 056pw-2010 7 126 161.7 7.5 111.9 832.3 15 391.3 26.7 7.9 68.7 3379.7 1 3 9 1 1
17 005pw-2010 7 122.7 161.7 7.6 97.9 745 16 391.7 24.3 7.7 76.2 2941.3 1 2 7 1 3
18 049pw-2010 7 120 163.3 6.6 108.4 652.6 15 408 33.2 7.2 94.3 3023.3 1 3 3 3 3
19 055pw-2010 7 115.7 161.7 6 99.7 604.7 14 389.7 30 8 78.1 3342.7 2 3 3 1 1
20 031pw-2010 7 119.3 163.3 6 104.1 624 15 393.7 28.3 8.3 75.7 2908.3 1 3 3 1 1
21 027pw-2010 7 121.3 163.3 7.6 102.5 781.3 13 401.7 33.3 7.3 96.7 2418.7 1 3 3 7 3
23 041pw-2010 7 120.7 165 7.4 96.5 722.4 14 308 19.4 8.1 63.2 3225.7 4 1 3 1 1
24 042pw-2010 7 112.7 161.7 6.3 99.4 629.7 15 386.7 28.7 8.7 89.8 2760 1 3 3 3 3
25 023pw-2010 7 119.3 163.3 6.3 98.4 611.7 12 404.7 32.7 7.4 90 3169 2 3 3 1 2
26 Emohay 7 88 141 6.7 75.7 528.3 8 266.7 36.1 7 92.3 4503 3 3 3 3 3
DTE-days to emergence, DTF-days to 50% flowering, DTM-days to 95%
maturity, LFW-leaf width (cm), LFL-leaf length(cm), SLA-single leaf area (cm2),
NOL-number of leaves per plant, PH-plant height (cm), HL-head length (cm),
HW-head width (cm), HWt-head weight(g), GRY -grain yield (g/plot), IEX-
inflorescence exertion, HS-head shape, GLC- glumes covering, GRC-grain color.
NB. Accession number 22 missed in all three replications and the trait
DTE is not included in all analysis because it is constant for all accessions.
Table 6. Table of analysis of variance (ANOVA)
|
Source of variation
|
|
Mean Squares
|
|
df
|
DTF
|
DTM
|
LFW
|
LFL
|
SLA
|
NOL
|
PH
|
HL
|
HW
|
HWt
|
GRY
|
|
Replication
|
2
|
0.04
|
287.5
|
0.14
|
59.69
|
2583.82
|
3.09
|
1435.88
|
9.75
|
4.85
|
3.18
|
1272149.00
|
|
Genotype
|
24
|
399.94*
|
273.61*
|
1.79
|
202.99*
|
25411.48**
|
12.62*
|
9751.24*
|
60.28**
|
3.29
|
313.88*
|
671061.10*
|
|
Error
|
48
|
1.94
|
17.92
|
0.52
|
12.44
|
4941.29
|
0.74
|
418.65
|
4.38
|
1.34
|
172.35
|
325204.40
|
|
R2
|
|
0.95
|
0.89
|
0.63
|
0.89
|
0.72
|
0.89
|
0.92
|
0.87
|
0.57
|
0.47
|
0.54
|
|
C.V
|
|
2.65
|
2.68
|
10.22
|
3.54
|
10.01
|
6.19
|
5.79
|
7.13
|
14.42
|
15.97
|
19.08
|
*, ** Significant at 0.05 and 0.01 probability level, R2=efficiency
of the model, CV= coefficient of variation, DTF =Days to
50% flowering, DTM=days to 95% maturity, LFW=leaf width, LFL=leaf length,
SLA=single leaf area, NOL=number of leaves per plant, PH=Plant height, HL=head
length, HW=head width, HWt=head weight, GRY =grain yield, df=degrees of freedom
Table
7.
Genetic parameters for eleven quantitative
traits in sorghum genotypes
|
Characters
|
N
|
R
|
Min
|
Max
|
Mean + SD
|
MSg
|
MSE
|
GV
|
PV
|
GCV
|
PCV
|
H
|
GA
|
GAM
|
DTF
|
75
|
41
|
87
|
128
|
113.64 +
11.7
|
399.94
|
1.94
|
130.27
|
133.31
|
10.1
|
10.2
|
97.8
|
5.78
|
5.1
|
|
DTM
|
75
|
40
|
130
|
170
|
157.83 +
10.4
|
273.61
|
17.92
|
85.23
|
91.2
|
5.9
|
6.1
|
93.5
|
2.06
|
12.7
|
|
LFW
|
75
|
4.8
|
4.8
|
9.6
|
7.07 + 0.96
|
1.79
|
0.52
|
0.42
|
0.6
|
9.2
|
11
|
70
|
1.39
|
19.7
|
|
LFL
|
75
|
41
|
71.8
|
112.8
|
99.61 + 8.7
|
202.99
|
12.44
|
63.52
|
67.66
|
8
|
8.3
|
93.9
|
16.83
|
16.9
|
|
SLA
|
75
|
437
|
458
|
895
|
702.31 +
107.3
|
25411.48
|
4941.29
|
8453.86
|
8470.3
|
13
|
13.1
|
99.8
|
220.96
|
31.5
|
|
NOL
|
75
|
9
|
8
|
17
|
13.88 + 2.2
|
12.62
|
0.74
|
3.96
|
4.24
|
14.4
|
14.8
|
94.3
|
4.20
|
30.3
|
|
PH
|
75
|
220
|
203
|
423
|
353.19 +
58.9
|
9751.24
|
418.65
|
3110.86
|
3250.4
|
15.8
|
16.2
|
95.7
|
116.35
|
33
|
|
HL
|
75
|
24.2
|
19
|
43.2
|
29.33 + 4.8
|
60.28
|
4.38
|
18.63
|
20.09
|
14.8
|
15.3
|
92.8
|
9.64
|
32.9
|
|
HW
|
75
|
7
|
5
|
12
|
8.04 + 1.4
|
3.29
|
1.34
|
0.65
|
1.09
|
10.1
|
13
|
59.7
|
1.77
|
22.1
|
|
HWt
|
75
|
73.8
|
47
|
120.8
|
82.19 + 14.6
|
313.88
|
172.35
|
47.18
|
104.63
|
8.4
|
12.5
|
45.1
|
13.6
|
16.6
|
|
GRY
|
75
|
2364
|
2174
|
4538
|
2988.6 +
680.4
|
671061.1
|
325204.4
|
115285.57
|
223687
|
11.4
|
15.9
|
51.6
|
724.31
|
24.33
|
DTF-days
to 50% flowering, DTM-days to 95% maturity, LFW-leaf width (cm), LFL-leaf
length (cm), SLA-single leaf area (cm2), NOL-number of leaves per
plant, PH-plant height (cm), HL-head length(cm), HW-head width(cm), HWt-head
weight (g), GRY-grain yield (g), N-number of observations, R-range, Min-minimum,
Max-maximum, MSg-mean square of genotype, MSE-mean square of error, SD-standard
deviation, GV-genotypic variance, PV-phenotypic variance, GCV-genotypic
coefficient of variation, PCV-phenotypic coefficient of variation,
H-heritability, GA-Genetic advance, GAM- genetic advance as percent of the mean
Table 8. Genotypic (above the empty
diagonal) and phenotypic (below the empty diagonal) correlation between 11 the
quantitative traits
|
Characters
|
DTF
|
DTM
|
LFW
|
LFL
|
SLA
|
NOL
|
PH
|
HL
|
HW
|
HWt
|
GRY
|
DTF
|
|
0.00
|
0.28
|
0.00
|
0.00
|
0.00
|
0.00
|
0.44*
|
0.01
|
0.99*
|
0.08
|
|
DTM
|
0.86**
|
|
0.35*
|
0.00
|
0.00
|
0. 00
|
0. 00
|
0. 89**
|
0. 00
|
0. 48*
|
0. 69**
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LFW
|
0.12
|
0.10
|
|
0.69*
|
0.00
|
0.51*
|
0.99**
|
0.52*
|
0.05
|
0.22*
|
0.11
|
|
LFL
|
0.78**
|
0.69**
|
0.04
|
|
0.00
|
0. 00
|
0. 00
|
0. 88*
|
0. 05
|
0. 92**
|
0. 01
|
|
SLA
|
0.50**
|
0.42**
|
0.81*
|
0.51**
|
|
0.00
|
0.00
|
0.47*
|
0.03
|
0.14
|
0.02
|
|
NOL
|
0.82**
|
0.73**
|
0.07
|
0.68**
|
0.39**
|
|
0.00
|
0.22*
|
0.03
|
0.91**
|
0.00
|
|
PH
|
0.78**
|
0.74**
|
0.00
|
0.68
|
0.32**
|
0.75**
|
|
0.02
|
0.02
|
0.05
|
0.10
|
|
HL
|
-0.08
|
0.01
|
0.07
|
0.01
|
-0.08
|
-0.14
|
0.25*
|
|
0.07
|
0.00
|
0.74*
|
|
HW
|
0.27*
|
0.33**
|
0.22
|
0.22
|
0.25*
|
0.23*
|
0.26*
|
0.20
|
|
0.30**
|
0.33**
|
|
HWt
|
0.00
|
0.08
|
-0.14
|
0.01
|
-0.17
|
-0.01
|
0.22
|
0.58**
|
0.12
|
|
0.63**
|
|
GRY
|
-0.19
|
-0.04
|
-0.18
|
-0.28
|
-0.25*
|
-0.32**
|
-0.18
|
0.03
|
-0.11
|
0.05
|
|
*-Significant at 5%, **-significant at 1% level of
significance, DTF-days to 50% flowering, DTM-days to 95% maturity, LFW-leaf
width, LFL-leaf length, SLA-single leaf area, NOL-number of leaves per plant,
PH-plant height, HL-head length, HW-head width, HWt-head weight, GRY -grain
yield
Table 9.
Estimates of direct (on diagonal) and indirect effects (off diagonal) of
different characters on grain yield
|
Effect of Character
|
Via Character
|
|
DTF
|
DTM
|
LFW
|
SLA
|
LFL
|
NOL
|
PH
|
HL
|
HW
|
HWt
|
rg
|
|
DTF
|
0.38
|
0.43
|
-0.23
|
-0.09
|
0.11
|
-0.14
|
0.66
|
0.00
|
-0.06
|
-0.01
|
0.38
|
|
DTM
|
0.36
|
0.45
|
-0.16
|
-0.09
|
0.11
|
-0.14
|
0.66
|
0.00
|
-0.07
|
-0.02
|
0.45
|
|
LFW
|
0.15
|
0.12
|
-0.58
|
-0.08
|
0.08
|
-0.08
|
0.21
|
-0.06
|
-0.04
|
0.03
|
-0.58
|
|
SLA
|
0.19
|
0.22
|
-0.27
|
-0.18
|
0.08
|
-0.09
|
0.31
|
-0.03
|
-0.06
|
0.04
|
-0.18
|
|
LFL
|
0.31
|
0.36
|
-0.33
|
-0.11
|
0.14
|
-0.13
|
0.56
|
-0.01
|
-0.05
|
0.01
|
0.14
|
|
NOL
|
0.32
|
0.38
|
-0.30
|
-0.10
|
0.11
|
-0.17
|
0.57
|
-0.05
|
-0.07
|
0.02
|
-0.17
|
|
PH
|
0.33
|
0.40
|
-0.16
|
-0.07
|
0.10
|
-0.12
|
0.76
|
0.07
|
-0.06
|
-0.05
|
-0.76
|
|
HL
|
0.00
|
0.01
|
0.12
|
0.02
|
-0.01
|
0.03
|
0.20
|
0.28
|
-0.02
|
-0.16
|
0.28
|
|
HW
|
0.17
|
0.23
|
-0.15
|
-0.08
|
0.05
|
-0.08
|
0.31
|
0.04
|
-0.15
|
-0.02
|
-0.15
|
|
HWt
|
0.01
|
0.05
|
0.09
|
0.04
|
-0.01
|
0.01
|
0.20
|
0.23
|
-0.02
|
-0.19
|
-0.19
|
Residual
Effect (R) = 0.63
DTF-days to 50% flowering,
DTM-days to 95% maturity, LFW-leaf width, LFL-leaf length, SLA-single leaf
area, NOL-number of leaves per plant, PH-plan eight, HL-head length, HW-head
width, HWt-head weight, rg-path
coefficient
Table
10. Clustering pattern of 25 sorghum genotypes
|
Clusters
|
No of genotypes
|
Genotypes included in the clusters
|
|
I
|
1
|
Emohay (the standard check)
|
|
II
|
4
|
012PW-2010, 001pw-2010, 003pw-2010 and 035pw-2010
|
|
III
|
8
|
041PW-2010, 021PW-2010, 056PW-2010, 047pw-2010, 005pw-2010, 020PW-2010,
044pw-2010 and 043pw-2010
|
|
IV
|
6
|
023PW-2010, 031PW-2010, 055PW-2010, 042PW-2010, 049PW-2010 and
045PW-2010
|
|
V
|
6
|
027PW-2010, 030PW-2010, 050PW-2010, 026PW-2010, 037PW-2010 and
033PW-2010
|
Table 11. Mahalanobis’s generalized
distance (D2) statistics of the 5 clusters of sorghum
|
Clusters
|
I
|
II
|
III
|
IV
|
V
|
|
I
|
0
|
889.53
|
595.38
|
872.91
|
878.03
|
|
II
|
|
0
|
309.61
|
22.659
|
90.299
|
|
III
|
|
|
0
|
291.1
|
289.88
|
|
IV
|
|
|
|
0
|
88.46
|
|
V
|
|
|
|
|
0
|
Table 12. Cluster mean of 11 quantitative
characters of sorghum germplasm
|
Characters
|
DFL
|
DYM
|
LFW
|
LFL
|
SLA
|
NOL
|
PH
|
HL
|
HW
|
HWt
|
GRY
|
|
I
|
88
|
141
|
6.7
|
75.7
|
528.3
|
8
|
266.7
|
36.1
|
7
|
92.3
|
4503
|
|
II
|
93.07
|
138.9
|
7.05
|
88.55
|
631.37
|
11
|
252.6
|
27.57
|
7.05
|
77.37
|
2954.6
|
|
III
|
119.92
|
162.26
|
7.38
|
102.97
|
760.65
|
14.75
|
353.25
|
25.83
|
8.21
|
74.2
|
3037.8
|
|
IV
|
118.16
|
162.21
|
6.13
|
101.95
|
619.31
|
14.33
|
392.56
|
30.05
|
7.78
|
84.78
|
2974.43
|
|
V
|
118.71
|
162.93
|
7.716
|
102.86
|
778.61
|
14.5
|
394.95
|
32.93
|
8.98
|
90.86
|
2672.43
|
DTF-days to 50% flowering,
DTM-days to 95% maturity, LFW-leaf width (cm), LFL-leaf length (cm), SLA-single
leaf area (cm2), NOL-number of leaves per plant, PH-plant height
(cm), HL-head length (cm), HW-head width (cm), HWt-head weight (g), GRY-grain
yield in gram per plot







Figure 1. A dendrogram showing the
clustering patterns of 25 sorghum landraces in north western BGRS, Ethiopia
(key to legend: for the local name of sorghum landraces, refer to Table 1).
ABBREVIATIONS/ACRONYMS
ANOVA Analysis of variance
CV Coefficient
of Variation
DAP Di ammonium phosphate
df Degrees
of freedom
GA Genetic
Advance in Absolute units
GAM Genetic Advance as percentage of the mean
GCV Genotypic
Coefficient of variation
IBC Institute
of Biodiversity Conservation
IBPGR International
Board for Plant Genetic Resources
ICRISAT International
Crop Research Institute for Semi-Arid Tropics
NCSS Number Cruncher Statistical System
PARC Pawe Agricultural Research Centre
PCV Phenotypic Coefficient of Variation
PV Phenotypic Variance
RCBD Randomized
Complete Block Design
SAS Statistical Analysis System
Understanding
the morphological variability correlated to the landraces distribution in the
area is an important tool for efficient development of crop genetic resources.
This characterization study using five qualitative and eleven quantitative
characters showed a range of variation in both qualitative and quantitative
characters.
Frequency distribution of the landraces into
different categories of qualitative traits indicated that there is a phenotypic
variability among the tested sorghum genotypes. The reason for the observed
high percentage of loosely headed landraces than the compact headed landraces
seems that there was targeted selection of materials for grain mould resistance
in the areas where the accessions collected among the local farmers. Because
the loosely headed sorghums have an adaptive trait (open panicle) which
facilitates quick drying of the panicle in areas of high rainfall and humidity,
thereby minimizing grain weathering due to fungal diseases such as grain mould
(Stemler et al., 1977; Rao et al., 1981, Thakur et al., 2008). The dominancy for
accessions with slightly exerted inflorescence may be because of their
suitability for harvesting. Among farmers and other people in the collection
areas white colour grain types make better quality ‘injera’ and porridge than
the other two grain types. Besides, they have better market price than the
other two sorghum types and that is why these white seeded sorghums have larger
land covers than brown and red seeded types of sorghum where the grains are
mainly used for making a local homemade alcoholic drink called ‘Tella’.
The significant mean square values obtained
from the analysis of variance suggests that differences existed between the
sorghum landraces for most characters, indicating that they are highly
variable. Eleven landraces gave better grain yield between 41.38 q/ha to 48.9 q/ha indicating the possibility
of identifying superior genotype to be selected among the landraces for the
breeding program. Genotype 021pw-2010 was the highest yielding (3565 g/plot or 48.9 q/ha) landrace, which is almost
less by 11 quintals when compared to the check variety (Emohay). The presence
of genotypes with good yield performance is an indication for the possibility
of developing high yielding varieties from the available materials for Pawe and
similar areas.
Generally, the studied genotypes showed range
of variability in their days to 50% flowering, days to 95% maturity, grain
yield, plant height, head length, head weight, head width, and in their leaf
character (leaf length, single leaf area, number of leaves and leaf width).
Therefore, morphological diversity among the sorghum landrace collections
implies the potential to improve the crop and the need to conserve these
resources.
The lowest genotypic and phenotypic
coefficients of variation was obtained for days to 95% maturity while the
highest PCV (15.8%) and GCV (16.2%) obtained for plant height. The significance
differences among the sorghum landraces in the investigation indicate the
presence of genetic variability in the material used and provide a good
opportunity for yield improvement on sorghum accessions. High ratios of the
phenotypic variance to genotypic variance for days to 95% maturity, leaf
length, leaf width, single leaf area, number of leaves per plant, plant height,
head length, head width, head weight and grain yield recorded indicating these
traits will be highly influenced by the environment. Since most tested sorghum
landraces in this research are very tall (above 3 meters), they may not be
suited for harvesting.
A high heritability estimates were recorded
for single leaf area (99.62%), days to 50% flowering (97.8%), plant height
(95.7%), number of leaves per plant (94.3%), leaf length (93.9%), days to 95%
maturity (93.5%) and leaf width (70%), indicating a high response to selection.
A lower heritability for head width (59.7%), head weight (45.1%) and grain
yield (51.6%) recorded. According to
Singh (1999), if heritability of a character is very high, say 80% or more, selection
for that character is easy, but the masking effect of the environment is high
on traits with low heritability (less than 40%). Thus, selection for single
leaf area, days to 50% flowering, plant height, number of leaves per plant,
leaf length, and days to 95% maturity will be easy for plant breeders among the
sorghum bicolor collections from the
four districts of Benishangul Gumuz regional state. Thus, relatively rapid
progress on the landraces improvement can be achieved through selection of
these traits.
The estimate of genetic advance (Table 4.3)
ranged from 1.39 cm (leaf width) to 724.31 g per plot (grain yield). That is
the resultant population obtained after crossing the best 5% of the materials
will produce a new population whose leaf width is increased by 1.39 cm, and
whose yield is better than the older population by 9.65 q/ha. Days to 95%
maturity, leaf width, leaf length, head length, head width, and grain yield,
which expressed high heritability with moderate GAM, appeared to be less
affected by environmental fluctuations and governed by both additive and
non-additive gene action, suggesting the possibility of improving these
characters through simple selection methods. Days to 50% flowering recorded the
lowest GAM (5.1%) indicating that this character will be affected by environment
and controlled by non-additive gene action.
The higher magnitude of genotypic correlation
coefficients than phenotypic correlation coefficients recorded in a correlation
of days to 50% flowering with leaf width, HL, and head weight; days to 95%
maturity with head length, head weight and grain yield; leaf length with leaf
width, head length, head weight, single leaf area, number of leaves per plant,
and grain yield; number of leaves per plant with leaf width, head weight and
head width, leaf width with plant height, head weight, and head length; single
leaf area with head length, and grain yield with head length, head width, and
head weight. Godbharle (2010) stated that traits with higher genotypic
correlation coefficients have inherent association. Therefore, the above
quantitative traits in this study with higher genotypic but lower phenotypic
correlation coefficient have inherent association.
The results of path
analysis revealed that days to 50% flowering, days to 95% maturity, leaf length
and head length showed positive direct effect with grain yield while leaf
width, single leaf area per plant, number of leaves per plant, plant height,
head width and head weight showed a negative direct effect on grain yield per
plot. Mahajan et al. (2011) stated
that head width, head length and plant height had greater importance in
improving grain yield. Thus, the present study revealed that the traits like
days to 50% flowering, days to 95% maturity, leaf length and head length had
greater importance. Hence, due consideration could be given to these characters
while planning a breeding strategy for increased grain yield. Most sorghum
collections were in the same cluster which might be due to close proximity of
collection sites and landrace exchanges could be common. Conservation and utilization of available sorghum landraces in
the collection areas must be given attention. Association of phenotypic variability with
genetic analysis will also help for better conclusions. The high performing
accessions of sorghum landraces screened in this study should further be
evaluated under a wide range of environments to find widely adapting landraces.
ACKNOWLEDGEMENTS
I would like to thank Pawe Agricultural Research Centre for
providing me a research site and seeds for the experiment. I wish to thank a
technical staff of PARC for their help in collecting the phenotypic data. My
gratitude thanks go to Dr. Tsige Genet (major
advisor), Dr. Tadesse Dessalegn (co -advisor), and Mr. Wasihun
Legesse (a researcher in PARC) for their critical
comments on the manuscript.
Abdi Adugna, Endashaw B., Zemede A. and Awgechew
Teshome
(2002). Patterns of
morphological variation of sorghum [Sorghum bicolor (L.) Moench]
landraces in qualitative characters in North Shewa
and South Welo. Hereditas. 137:161–172.
Abe
Shegro(2010).
Biodiversity in plant,
grain and nutritional characteristics of Sorghum [Sorghum bicolor (L.) Moench] accessions from Ethiopia and South
Africa, PhD thesis, University of the Free State, Bloemfontein, South Africa,164pp.
Addisu,
G. A. (2011). Heritability and genetic advance in recombinant inbred lines for
drought tolerance and other related traits in sorghum (Sorghum bicolor).
Continental Journal of Agricultural Science, 5 (1), 1 - 9
Adugna Asfaw (2007). The role of introduced
sorghum and millets in Ethiopian agriculture. J SAT Agric
Res. 3(1)
Allard, R.W (1960). Principles of plant breeding.
John Wiley and Sons, New York.
Amsalu
Ayana and Endashaw Bekele (1998). Geographical
patterns of Morphologic variation in Sorghum (Sorghum bicolor (L) Moench) germplasm from Ethiopia and Eritrea. Hereditas.129:195-205.
Amsalu Ayana (2000). Genetic diversity in sorghum
germplasm from Ethiopia and Eritrea, PhD dissertation, University of Addis
Ababa, Ethiopia.
Ashante J.A. (1995).
Sorghum grain quality and utilization, J. Afr. crop Sci. 3:231-240.
Bello D., Kadams A.M., Simon S.Y. and Mashi D.S. (2007). Studies on genetic variability in cultivated sorghum [sorghum
bicolour (L.) Moench] cultivars of Adamawa
state, Nigeria. American-Eurasian J. Agric. & Environ. Sci. 2:297-302.
Bohra P., Phal P.S.
and Allah R. (1986). Association analysis for yield
and quality traits in sorghum crop improvement. J. Agri. Sci.
12(2):89-93.
Burton
G.A. (1952). Estimating Heritability in tall fescu
(Festuca arundincea)
from replicated clonal material. J. Agro. 45:481-487.
Deepalakshmi A.J. and Ganesamurthy K. (2007). Studies on Genetic variability and character association in kharif
sorghum [sorghum bicolour (L) Moench], Indian J. Agric. Res. 41:177–182
Dewey, D.R. and Lu,
K.H. (1959). A correlation and path analysis of components of crested wheat grass
seed production. J. Agro. 51(6), 515-518.
Doggett H. 1988. Sorghum long mans. 2nd
Edition, London.
Durrishahwar, Muhammad N., Hidayat-ur-Rahman,
Ihteramullah, Irfan A. S.,
Farhan A., Syed M, Ali S. and Nasir M. (2012). Characterization of sorghum
germplasm for various morphological and fodder yield parameters. Afr. J.
Biotech. 11(56):11952-11959
Elangovan
M., Prabhaker D. and Chandra S. R. (2007). Characterization and Evaluation of Sorghum [Sorghum bicolor (L.) Moench] Germplasm, J. Agric. Sci. 20(4):840-842.
Ezeaku I.E. and Mohammed S.G. (2006). Character association and path analysis in grain sorghum, Afr. J. Biotech,
5 (14):1337-1340.
Falconer and Mackay (1961). How and when
selection experiments might actually be useful. Integ. Comp. Biol.
45:391-104.
Falconer
D.S. (1981). Introduction
to quantitative Genetics. 2nd Edition.
Longman Inc., New York.
Geremew Gebeyehu (1993). Morpho-agronomic characterization of sorghum land race s from Gambella.
MSc Thesis. Alemaya University, Ethiopia.
Godbharle A.R.,
More A.W. and Ambekar S.S. (2010). Genetic
Variability and Correlation Studies in elite ‘B’ and ‘R’ lines in Kharif
Sorghum. Electronic Journal of Plant Breeding.
1(4):989-993.
Gomez A.K.
and Gomez A.A. (1984). Statistical procedures for agricultural research. 2nd Edition, John Willey and Sons Inc., Neyork, 680 pp.
Govindaraj M., Shanmugasundaram P and Muthiah
A.R. (2010).
Estimates of genetic parameters for yield and yield attributes in elite lines
and popular cultivars of India’s pearl millet. Afr. J. Agric. Res. 5(22):3060-3064.
Habindavyi
Esperance (2009). Morphological characterization of sorghum (Sorghum
bicolor) diversity in Burundi. MSc Thesis. University of Uppsala, Uppsala. Burundi. 48pp.
House L.R. (1985). A guide to Sorghum Breeding.
2nd Edition, International crops research Institute for the Semi-Arid
Tropics, Patancheru, India. 206pp.
IBC (2008). Second Country
report on the State of PGRFA to FAO. Addis Ababa, Ethiopia
IBPGR/ICRISAT. (1993).
Descriptors for sorghum [Sorghum bicolor (L.) Moench]. International Board
for Plant Genetic Resources, Rome,
Italy; International Crops Research Institute for the Semi-Arid Tropics, Patancheru, India.
Jalal A., Al-Tabal, Ahmad H. and AL-Fraihat. (2011). Genetic
variation, Heritability, phenotypic and Genotypic Correlation studies for yield
and yield components in promising Barley Genotypes, Journal of
Agricultural Science. 4(3):193-210.
Mahajan R.C., Wadikar P B.,
Pole S P. and Dhuppe M.V. (2011).
Variability, Correlation and Path analysis studies in sorghum. J. Agric. Sci. 2:101-103.
Mahdieh Dalkani, Abbas Hassani
and Reza Darvishzadeh (2012). Determination of the genetic variation in Ajowan (Carum Copticum L.) populations using multivariate
statistical techniques. J. Agro. 43(4): 698-705.
Mehmoods, Bashira A., Ahmad A., Z. Akram (2008).
Molecular characterization of regional sorghum bicolor varieties
from Pakistan. Pak. J. Bot., 40(5): 2015-2021
Melakhail Mengesha (1975).
Crop germplasm
diversity and resource in Ethiopia. In: Crop genetic resources for
today and tomorrow. Cambridge University press, Cambridge, pp.433-465.
Melakhaile
Mengesha and Rao P. (1982). Current situation and future of sorghum in the eighties. Proceedings. The international Symposium, Patacheru, A.P. India, ICRSAT. pp. 323-333.
Melaku Worede (1988). Diversity and genetic
resources base. J. Agric. Sci. 10:39-52
Miller P.A., J.C.
Williams H.F. Robinson and Comstock R.E. (1958). Estimates
of genetic and environmental variance and covariance and their implication in
selection. J. Agro. 50:126-131.
NCSS (2004). Number Cruncher Statistical Systems, Dr. Jerry L. Hintze, 329 North
1000 East, Kaysville, Utah 84037, Canada.
Negash Geleta (2003). Genetic
Variability and Associations among yield and yield relate d traits in sorghum
[Sorghum bicolor (L.) Moench] Landraces of western Ethiopia.
MSc Thesis, Alemaya University,
Ethiopia.
Pascal
Institute Software solution (1994) GenRes, a statistical package for genetic
researchers. Version 3.11.
Rangswamy R. (1995). A text book of Agricultural
statics. Willey Eastern Ltd. Ney Delhi.
496pp.
Rao P.,
K.E. and M.H. Mengesha (1981). A pointed
collection of Zera zera
sorghum in the Gambella area. http://www.worldcat.org-html. 30/march/2012.
SAS Institute (2008).
Statistical Analysis Software version 9.2. SAS
Institute Inc., USA.
Singh P. and
Narayanan S.S. (1993). Biometrical techniques in plant breeding. Kalyani
Publishers, New Delhi.
Singh, B.D. (1999). Plant Breeding, principles
and merhods. 4th Edition. Kalyani
publishers, New Delhi, India. Pp.639
Solomon Benor and Lemlem
Sisay. (2003). Folk classification of sorghum [Sorghum bicolor (L.) Moench] landraces and its ethnobotanical implication. Etnobiología. 3:29-41
Stemler A., B. L.,
Harlan J.R. and De Wet J., M.J. (1977). The sorghum of
Ethiopia. J. Econ. Bot. 31:446-460.
Stickler F.C.S.
Wearden A.W. (1961). Leaf area determination in grain
sorghum. J. Agro. 53:187-188
Taylor S.
L., M. E. Payton & W. R. Raun (1999).
Relationship between mean yield coefficient of variation, mean square error,
and plot size in wheat field experiments, Communications in Soil Science and
Plant Analysis, 30:9-10, 1439-1447
Thakur
R.P., Rao V.P., and Sharma R. (2008). Characterization of
grain mold resistant sorghum germplasm accessions for physio-morphological
traits. Journal of SAT Agricultural Research. 6:1-7.
Vittal R., Ghosh
N., Weng Y., Stewart B.A. (2010). Genetic diversity among Sorghum
bicolor (L.)
Moench genotypes as revealed by prolamines
and SSR markers, Journal of Biotech Research, 2:101-111
Wasihun Legesse (2007). Agro-morphological characterization of Sorghum (Sorghum bicolor (L) Moench Landraces from Metekel
area, MSc Thesis. Hawasa University,
Ethiopia. 104pp.
Wright S. (1921). Systems of mating Genetics.
6:111-178.
Yilma Kedede (1991). The role of Ethiopian
sorghum germplasm resource in the national breeding program. In: Englkans J., G. Hawks and Melaku Worede (Eds.), Plant genetic resource of Ethiopia. Cambridge
University Press, Cambridge. pp. 315-322.
|
Cite
this Article: Gedifew G; Tsige G (2019).
Morphological Characterization and Evaluation of Sorghum [Sorghum bicolor
(L.) Moench] Landraces in Benishangul
Gumuz, North-western Ethiopia. Greener Journal of Agricultural
Sciences 9(1): 37-56, http://doi.org/10.15580/GJAS.2019.1.123118187.
|











