INTRODUCTION
Culicidae are responsible for the
transmission of pathogens that they can inoculate during their blood meal. They
represent, therefore, a real public health problem. Among these mosquitoes,
some are a source of nuisance difficult to bear. This is the case of Culex pipiens Linné,
1758, widespread in the world. It is present in tropical and temperate zones
(Weill et al. 2003). In Benin, malaria is the leading cause of attendance in
health facilities with a frequency of 47.6% in children under 5 and 41.7% in adults
(SNIGS, 2012). The risk of transmission is not clear. It varies over time and
space. According to the work of the Francophone Virtual Medical University
(UVMF) (2014), the spatial distribution of malaria transmission varies from one
area to another. The environment is a major determinant of the biodiversity of
malaria because of the vectorial nature of the transmission of Plasmodiums and
the bioecological preferences of the vectors. This biodiversity depends on
several factors associated with the development of mosquitoes and indirectly
with the transmission of Plasmodiums. These are the seasonality, distribution
and quantity of rainfall, temperature, humidity, altitude, the presence of
surface water or vegetation, as well as certain anthropogenic factors
(agricultural activities, irrigation, deforestation, urbanization, construction
of roads or dams).
Currently, mosquitoes, vectors of
these diseases, evolve in natural ecological systems and those modified by
humans (Makanga, 2016). Indeed, the development of oil palm plantations in
Gabon, particularly in the Mouila region, has led to a modification of the
natural landscapes and the impoundment of palm groves (Ndjimbi, 2013). These
new conditions, favorable to larval development and the proliferation of
mosquitoes, have led to a sharp increase in malaria cases in this region
(Koumba et al., 2018a; 2018b).
To deal with this problem of
mosquitoes vectors of pathogens, the World Health Organization (WHO) recommends
the establishment of vector control focused on the sanitation of the living
environment, the distribution of insecticide-impregnated mosquito nets
Long-Lasting Acting (LLIN) and Indoor Residual Insecticide Spraying (PNLP,
2010; Badolo et al., 2012; Abagli et al., 2014).
However, these interventions require a better knowledge of the bioecology of
vectors.These factors are at the origin of the appearance and the persistence
of breeding sites, the speed of larvae development, the survival of adults -
and therefore their density - or the rate of development of the parasite in
vector Anopheles (extrinsic cycle). They can therefore explain the diversity in
the level of malaria transmission from one area to another and from one season
to another (Bio Bangana, 2013).
Many mosquito breeding sites are
produced in both the northern and southern departments of the country due to
poor environmental management and insufficient water drainage (Akogbéto, 2005).
Environmental sanitation is therefore a factor in controlling malaria. Taking
all of the above into account, it is important to know the factors responsible
for the diversity and intensity of malaria.
In addition, spatial analysis is a
methodological approach making it possible to characterize a phenomenon indexed
by geographic coordinates with a view to describing it, explaining it and
modeling its behavior in space and / or time, all this in the aim of
identifying the tendency to form particular structures thus leading to the
formulation of hypotheses and to decision making. Risk mapping then makes it
possible to identify the vulnerabilities to which the risk areas are subject,
and therefore to prioritize the control actions in a hierarchical form. The
rainy season remains par excellence the favorable period for the proliferation
of malaria vector mosquitoes; the hot period at the end of the rainy season
sees a strong occurrence of malaria with a peak in October, whatever the
epidemiological facies. It is within this framework that the present study was
conducted and its objectives are: to identify the different sites colonized by
Anopheles, Culex and Aedae according to the seasons and geographical
specificities. Thus, the results of this
study can be used in the potential use of complementary control methods based
on the control of larval stages in the department of Alibori in Benin
MATERIALS AND
METHODS
Study
sites
The region of Alibori is characterized
by a Sudanese climate by a Sudano-guinean climate,
with a single dry season (December to May) and a single rainy season (June to
November). The annual average rainfall varies between 700–1200 mm, respectively
in Alibori region. The average monthly temperature varies between 23 and 40 °C
(INSAE, 2013).
The study was carried
out in two health zones composed of three districts in northen Benin:
Kandi-Gogounou-Ségbana health zone in Alibori province (Fig. 1). These sectors
are selected by the National Malaria Control Program (NMCP) based on
epidemiological, ecological, environmental and socio-economic criteria to extend
Indoor Residual Spraying (IRS) operations from 2017. The region's crop
diversity includes yams, sorghum, maize, millet, cowpeas, cassava, beans and
groundnuts for food-producing crops and cotton, shea and cashew for cash crops.
Collecting and processing cashew and shea are the main sources of income for
the populations. Kandi-Gogounou-Ségbana health zone situated in southern
Alibori provincecovers about 12,943 km2. Long- lasting insecticidal
nets (LLINs) are the main tool for malaria prevention in these districts.
Sampling: choice of study sites
For sampling mosquito
larvae, we used the "dip-ping" method (Rioux et al. 1965b; Subra
1971; Cro-set et al. 1976); Coffinet et al. 2009). This method consists of
collecting a one-liter dipper of water (c) from several locations within the
site without repetition. Using this method, we took a series of 5 samples and
calculated the average density of larvae per sample. This number is an estimate
of the average larval density per liter. The
identification was carried out with the help of the identification keys of
Rioux (1958) and Senevet and Andarreli (1959) which have largely contributed to
the knowledge of the Mediterranean culicid fauna. This identification was then
confirmed using the software on Culicidae of Mediterranean Africa designed by Brunhes
et al. (1999). Mosquito larvae surveys were
carried out in the three districts under IRS. A sampling plan by level was set
up by associating each municipality with an unequal weighting. In each
municipality, we randomly selected 50% of the arrondissements and in each
arrondissement, another level of selection which randomly took 25% of the
villages since the unequal weighting was also associated with each arrondissement.
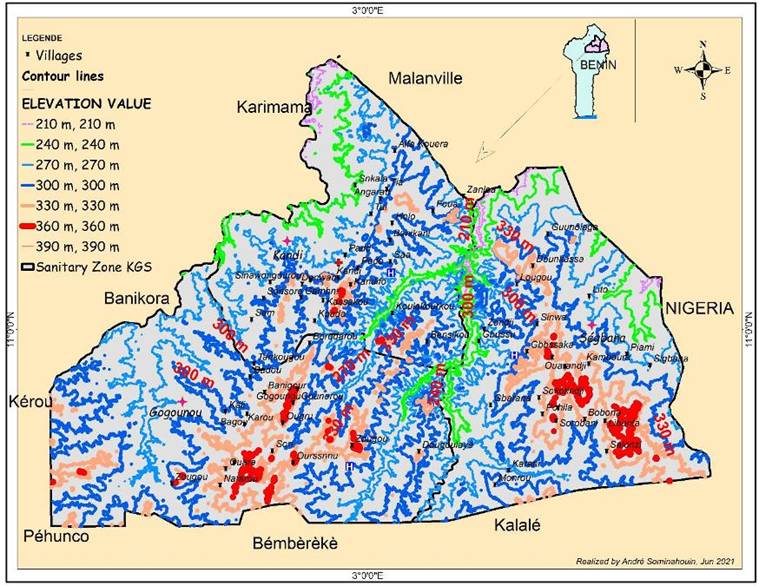
Figure 1 shows the municipality and the districts of
the sites surveyed. The districts indicated in this figure were chosen
respectively in the communes of Kandi, Gogounou, Ségbana. The objective of these
weightings is to satisfy the condition of representativeness of our sample.
DISCUSSION
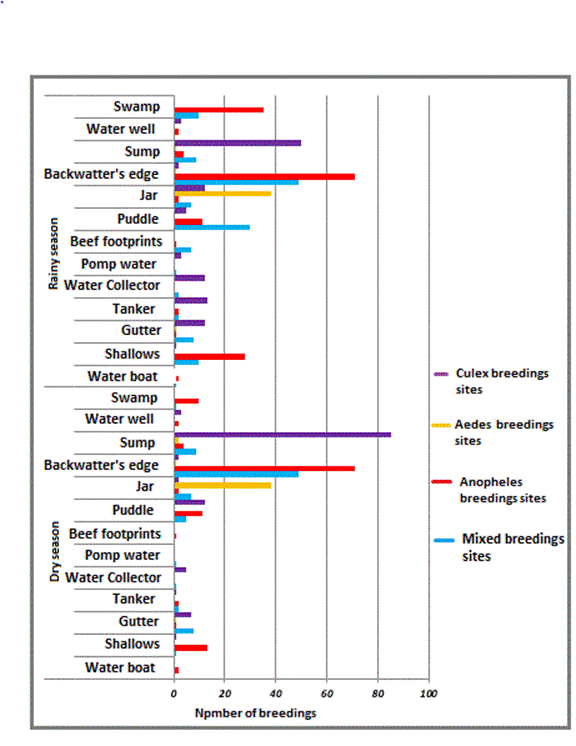
The larval surveys carried out in this
work showed that the study area has a diversity of breeding sites. The majority
of these breeding sites have been created by humans as a result of their
activities (agricultural activities, artificial water surfaces, etc.). These
roosts, especially those with at least one larva, are found in the immediate
environment of human populations (less than 400 m from dwellings) as also
observed in Benin by Akogbeto (2000). In addition, these sites are conducive to
the development of mosquitoes of the genera Culex, Aedes and Anopheles which
are major vectors of many pathogens responsible for many pathologies including
malaria, chikungunya, filariasis, dengue, yellow fever, the zika virus.
(Rodhain, 2015). These results are similar to those of Tia et al. (2016) who
showed the responsibility of the inhabitants in the establishment of conditions
conducive to the development and maintenance of mosquitoes through the creation
of their larval habitats. The classic sites listed are sunny, clean, clear
water points (Mouchet et al., 2004). In northern
Benin, these are puddles, temporary ponds, earthen holes or brick quarry holes.
These lodgings bear witness to poor sanitation of the living environment due to
the laxity of the competent public services and / or the negligence of the
local population. Likewise, the observation of rice fields, lowlands used for
market gardening and watering wells shows the dynamism of urban agriculture in
the locality, a cause of urban malaria of the same importance as the more
classic rural malaria. . In the departments of Alibori and Atacora, in rural
areas, the classic lodgings observed are the lowlands that surround the village
and used for rice cultivation and whose water is kept in jars for domestic use.
In addition to these lowlands, the presence of many temporary pools or puddles
of anthropogenic origin constitute a source of culicidian nuisance for the
populations and a risk factor for malaria in this locality. There is a need to raise awareness about sanitation of the living
environment and the use of LLINs. Conserving water from the rains or from the
aforementioned sources makes it possible to reduce the physical effort imposed
on women, that of traveling long distances to meet the family's water needs. In
addition to the cisterns, An. gambiae s.l. was also collected in barrels, animal
drinkers (duck, chicken) with greenish and shaded waters. We observe that An. gambiae s.l was collected as well
and with the same abundance in the classic roosts as in the atypical roosts
where the water is generally cloudy (cistern, barrel,) or polluted (animal
drinking troughs) like (Karch et al., 1993; Coffinet
et al., 2009; Noumi et al., 2012). This observation reveals that these
deposits, in particular the domestic water storage jars, are no longer the
prerogative of Ae . aegypti, but
that the presence of An.gambiae in
these atypical sites should now call on those responsible for malaria control
programs to take it into account in vector control projects.
CONCLUSION
The results obtained during this study
show that mosquitoes thrive in all types of water points but prefer artificial
water surfaces. The majority of positive larval habitats were found in the
immediate environment of human populations. The maximum number of breeding
sites and larvae was recorded in the rainy season with an abundance of larvae
of the Culicinae subfamily compared to those of the Anophelinae subfamily. This
larval production is dependent on the season, human practices and the length of
time the water is stored in artificial containers. The rainy season is when
mosquitoes develop due to the presence of rainwater puddles, the main
ecological preference of mosquitoes of the genus Anopheles. So after the rains
and floods, think about mosquitoes by taking measures to limit the
proliferation of mosquitoes. The central state must increase village water
supplies, make them geographically and financially accessible to all. This will
make it possible to limit the use of rainwater and marshes towards homes and
therefore that of the nuisance of mosquitoes. Local authorities should organize
communication sessions with the population for behavior change. The
intervention of municipal authorities through community radio channels can
significantly reduce the risk of the spread of mosquitoes and diseases. It is
also necessary to backfill the depressions of the tracks, to the cleaning of
the gutters. Such an action allows the suppression of hen nests which are true
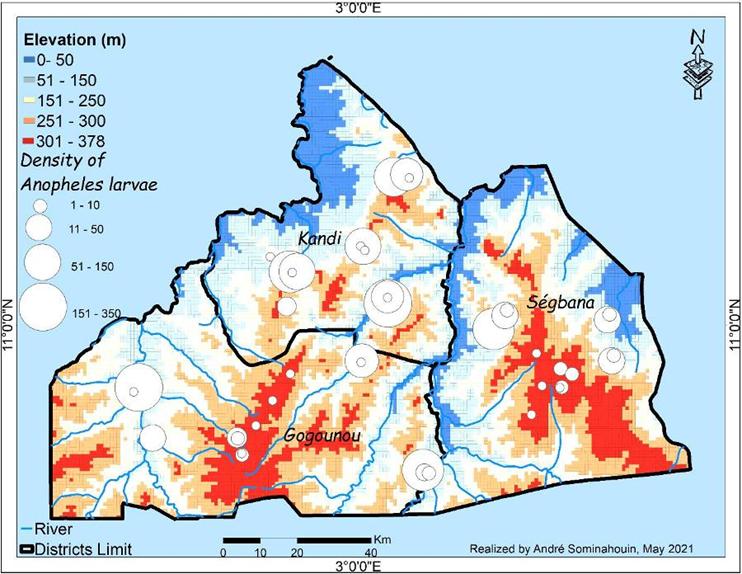
breeding grounds for mosquitoes. The vulnerability maps obtained from the
addition of the physiographic layers considered in each model show the
variability of vulnerability levels for each genus of Culicidae. These results
show that more than half of the territory of the Kandi-Gogounou-Segbana health
zone offers good ecological conditions for mosquito development, thus exposing
more than half of the population to diseases caused by these mosquitoes.
Competing
interests
The
authors declare having no competing interests.
Acknowledgment
Funding
This study was financially supported by
the Centre de Recherche Entomologique de Cotonou
Availability
of data and materials
The data supporting the conclusions of
this article are included within the article. The raw data used and/or analyzed
in this study are available from the corresponding author upon reasonable
request.
Authors’ contributions
ASS, GGP, RA and MCA designed the study.
ASS, MA, FA and MCA participated in the design of the study. ASS, EO SA, FA and
AAS collected entomological data. ASS, SA, FA and AAS carried out bioassays and
laboratory analysis. ASS and MCA drafted the manuscript. FA, AS, RA, AF, KC and
MCA critically revised the manuscript for intellectual content. All authors
read and approved the final manuscript.
Ethics
approval and consent to participate
Not applicable.
Consent
for publication
Not applicable.
Bibliographic
References
Abagli
AZ, Alavo TBC, Brodeur J. 2014. Microorganismes entomopathogènes, prédateurs et
parasites des moustiques : Perspectives pour la lutte raisonnée contre les
vecteurs du paludisme en Afrique subsaharienne. International Journal of
Biological and Chemical Sciences, 8(1): 340-354. DOI:
http://dx.doi.org/10.4314/ijbcs.v8i1.29.
Akogbeto
M, 2000. Impact des modifications de l’environnement et du degré de salinité
des gîtes d’eau saumâtre sur la dynamique de population d’An. melas, vecteur du paludisme dans le milieu côtier lagunaire
du Benin. Cahier d’étude de recherches francophones/Agriculture. Colloque
international « Eau et Santé »,9 (5): 422-427.
Akogbeto
M, Nahum A. Impact des moustiquaires imprégnées de deltaméthrine sur la
transmission du paludisme dans un milieu côtier lagunaire, Bénin. Bull Soc PatholExot 1996; 89: 291–8.
Badolo
A, Ilboudo-Sanogo E, Sanon A, Ouédraogo AP. 2012. Evaluation de la protection
personnelle contre les Anophelinae par utilisation de moustiquaires détériorées
imprégnées de répulsifs. International Journal of Biological and Chemical
Sciences, 6(1): 237-247. DOI: http://dx.doi.org/10.4314/ijbcs.v6i1.20.
Brunhes J.,
Rhaim A., Geoffroy B., Angel G. & Hervy J-P., 1999. Les Moustiques de
l'Afrique méditerranéen, logiciel d'identification et d'enseignement, I.R.D.
édition.
Coffinet
T, Rogier C, Pages F. 2009. Evaluation de l’agressivité des anophèles
et du risque de transmission du paludisme, méthodes utilisées dans les armées
françaises. Medecine Tropicale, 69: 109- 122.
INSAE, RGPH4 2013. Cahiers des villages et quartiers
de ville du département de l’Alibori; 2016. http://www.insae-bj.org/recensement-population.html/enquêtes-.
Makanga PT, Schuurman N, von Dadelszen P, Firoz T. A
scoping review of geographic information systems in maternal health. Int J
Gynaecol Obstet. 2016 Jul;134(1):13-7. doi: 10.1016/j.ijgo.2015.11.022. Epub 2016 Apr 1. Erratum
in: Int J Gynaecol Obstet. 2016 Dec;135(3):388. PMID:
27126906; PMCID: PMC4996913.
Makungu C, Stephen S, Kumburu S, Govella NJ, Dongus S,
Hildon ZJ-L, et al. Informing new or improved vector control tools for reducing
the malaria burden in Tanzania: a qualitative exploration of perceptions of
mosquitoes and methods for their control among the residents of Dar es Salaam.
Malar J. BioMed Central; 2017; 16: 410.
https://doi.org/10.1186/s12936-017-2056-9 PMID: 29020970.
Mouchet
J, Carnevale P, Coosemans M, Julvez J, Manguin S, Richard-Lenoble D, et
Sircoulon J, 2004. Biodiversité du paludisme dans le monde. Editions John
Libbey Eurotext., Paris. 428 p.
Ndjimbi
F. 2013. Les populations Gabonaises face à l’insécurisassions foncière, étude
d’impact des plantations agro-industrielles de palmiers à huile et d’hévéa sur
les populations du Gabon. ONG Brainforest, p. 72.
Noumi,
2012, Spectroscopic study of
hyperon resonances below KbarN threshold via the (K−, n) reaction on Deuteron ² J-PARC
E31 proposal, (http://j-parc.jp/NuclPart/pac_0907/pdf/Noumi.pdf)
PNLP.
2010. Statistique épidémiologique du Gabon. Rapport annuel d’activités.
Ministère de la Santé, Libreville, p. 30.
Karch
S, Garin B, Asidi N, Manzambi Z, Salaun JJ, Mouchet J. Moustiquaires impregnées
contre le paludisme au Zaire. Ann. Soc. Belge Méd. trop. [Internet]
1993;73:37-53. Available
from:http://lib.itg.be/open/ASBMT/1993/1993asbm0037.pdf.
Koumba
AA, Zinga-Koumba CR, MintsaNguema R, Sevidzem SL, Djogbenou LS, Akono PN, Ketoh
GK, Faye O, M’batchi B and Mavoungou JF. 2018b. Identification of the knockdown
resistance (Kdr) mutations in Anopheles gambiae s.l. in the Mouila area, South
west Gabon. Journal of Entomology and Zoology Studies, 6(3): 602-607.
Koumba
AA, Zinga-Koumba CR, MintsaNguema R, Bi Zahouli JZ, Ovono AM, Souza A, Ketoh
GK, Djogbenou LS, M’batchi B, Mavoungou JF. 2018a. Preliminary evaluation of
the insecticide susceptibility in the culicid fauna, particularly malaria
plasmodium and arbovirus vectors in the region of Mouila, South-west Gabon.
Indian Journal of A. A.KOUMBA et al. / Int. J. Biol. Chem. Sci. 12(4):
1754-1769, 2018 1769 Medical Research and Pharmaceutical Sciences, 5(4): 105-117.
DOI: 10.5281/zenodo.1236958.
Rodhain
F. 2015. Le parasite, le moustique, l’homme et les autres : Essai sur
l’épidémiologie des maladies à vecteurs, Edition Dorcas, p. 440.
Senevet
G et Andarelli L., 1959. - Les moustiques cle l’Afrique du Nord et du Bassin
méditerranéen. Les genres Czzlex, Llranotaenia, Theobald+, Orthopodomyia ‘. et
Manson,ia.‘Encycl. ect. P. Lechevalier, Alger, 37, 383
p.
SNIGS
(2012) : Annuaire des statistiques sanitaires 2011, Ministère de la Santé,
Direction de la Programmation et de la Prospective, 80p.
Tia
E, Gbalegba NGC, M’bra KR, Kaba A, Boby OAM, Koné M, Chouaibou M, Koné B,
Koudou GB. 2016. Etude du niveau de production larvaire d’Anopheles gambiae
s.l. (Diptera : Culicidae) dans les différents types de gîtes à Oussou-yaokro
au Centre-Ouest et à Korhogo, au Nord (Côte d’Ivoire). Journal of Applied
Biosciences, 105 : 10170-10182. DOI : http://dx.doi.org/10.4314/jab.v105i1.13.
Tia
E, Gbalegba NGC, M’bra KR, Kaba A, Boby OAM, Koné M, Chouaibou M, Koné B,
Koudou GB. 2016. Etude du niveau de production larvaire d’Anopheles gambiae
s.l. (Diptera : Culicidae) dans les différents types de gîtes à Oussou-yaokro
au Centre-Ouest et à Korhogo, au Nord (Côte d’Ivoire). Journal of Applied
Biosciences, 105 : 10170-10182. DOI : http://dx.doi.org/10.4314/jab.v105i1.13.
UVMF,
2014. Paludisme. ,. Association Française des Enseignants de Parasitologie et
Mycologie (ANOFEL), 27p.