INTRODUCTION
Lead is a heavy metal element exists widely
in the environment and it has neurotoxin effects. In contrast to trace elements
such as iron and zinc, lead has no know beneficial effects in the human body
(Zheny et al., 2008). Grant and Davis (1989) reported that lead
inhalation is a global issue as lead mining and lead smelting are common in
many countries and long-term exposure to lead can cause nephropathy and
colic-like abdominal pains, weakness in finger and wrists or ankles. As well,
Hou et al, (2013) stated that lead exposure causes small increase in
blood pressure, anemia, damage the brain and kidneys in adults or children and
ultimately cause death. Inhalation is the major pathway of the exposure
especially for smelters and workers in lead related occupation. It has been
reported by Cohen et al. (1981) that almost all inhaled lead is absorbed
into the body, while the rate of absorption for ingested lead is 20 – 70% and
children absorb more than adults and petrol sniffing in addition to other
volatile substances such as spirit-base glues are also classified as toxic
lead-containing substances that affect and altering the mind and mood of
sniffers and leaded petrol and the volatile substances are highly lipophilic
and are rapidly absorbed through the body and cross the blood brain barriers of
the sniffers resulting in many complications. Brady (1992) believed that the
existence of sniffing phenomenon is significantly associated with less family
support. In Sudan, problems of migration of people from neighboring countries
as well as the civil war in south and west of Sudan are behind
the reason of homeless refugees. In Khartoum City, homeless children mainly
sniff benzene and selesion (a substance used in tire repair) and they prefer
selesion due to low price comparing to benzene. Hence, the objective of this
study is to investigate the effect of addiction to sniffing lead-containing
substances on blood biochemical measurements of adolescents in Khartoum City,
Sudan.
MATERIALS AND METHODS
Area of the study:
The study was carried out in
Khartoum State/Sudan during 2013 to investigate the effect
of sniffing of petrol and other volatile substances on biochemical and
behavioral status of adolescent sniffers. Three rehabilitate centers of
homeless were selected namely Tayba (south of Khartoum/for boys, Bashair
(Omdurman) for girls Rashad (west of Khartoum) for both young
boys and girls).
Sample size:
A total of 120 sniffers were selected from
these three rehabilitate centers, 40 participants from each, in addition to 40
non-users as a control subjects. Age of
sniffers range between 6 and 18 years. Blood samples were then
collected from the selected sniffers as well as the control to determine lead
and calcium concentration, liver function (protein, albumin, bilirubin, AST and
ALT) and lipid profile (cholesterol and triglyceride).
Blood sample collection:
A 5 ml of blood from peripheral vein of
sniffers (boys and girls) were collected in heparinized container as
anticoagulants in order to determine the biochemical parameters. For sample
collection, disposable syringes, heparin tubes, cotton, and ethanol were used.
Determination of lead in blood serum and
plasma:
The BC-5 Analysis was used for the
determination of lead in blood serum and plasma. The blood sample was diluted
in deionized water and the analysis was then
performed against standards prepared in glycerol to approximate the viscosity
characteristics of the diluted samples. Normal serum levels estimated as µg%
(Pb 10 – 20) or mg/dl (0.001- 0.002). For determination of serum lead, the
sample was diluted1:5 with deionized water. The condition listed in the
“Standard Conditions” section was used to determine the concentration of lead.
Lead Standards were prepared by diluting the lead stock standard solution
described in the Standard Conditions” for lead with 5% (v/v) glycerol solution
were also used as a blank solution when determining lead concentration
(Butrimovitz and Anal, 1977).
Calcium determination:
Calcium in serum or plasma was stable for 10
days at 2-8oC. Anticoagulants other than heparin should not be used.
The calcium concentration in the sample was calculated using the method
described by Cheesbrough (2006).
Protein determination:
Protein in the sample reacts with copper (II)
ion in alkaline medium forming a colored complex that can be measured by
spectrophotometer. Serum or heparinized plasma was collected by standard
procedures. Stable for 8 days at 2 – 8oC. Anticoagulants other than
heparin should not be used. The protein concentration in the sample was
calculated using the method described by Cannon et al. (1974).
Albumin determination:
The serum albumin
concentration was determined using modified bromocresol green colorimetric
method as described by Doumas et al. (1971). Measurement of albumin is
based on its binding to the indicator dye bromocresol green (BCG) in pH 4.1 to
form a blue – green colored complex. The intensity of the blue – green colorist
directly proportional to the concentration of albumin in the sample. It is
determined by monitoring the increase in absorbance at 623 nm.
Absorbance of the sample and standard were read against reagent
blank at 505 nm. The activity of enzyme was determined from the absorbance
table U/L.
Cholesterol determination:
Very low density lipoproteins (VLDL) and low
density lipoproteins (LDL) in the sample were precipitated with phosphotungeslate
and magnesium ions. The supernatant contains high density lipoproteins (HDL).
The HDL cholesterol was then spectrophotometrically measured according to
Cheesbrough (2006).
Triglycerides determination:
Triglycerides in the sample originates by
means of the coupled reactions described below, a colored complex that can be
measured by spectrophotometer.The triglycerides
concentration in the sample was calculated using the method described by
Cheesbrough (2006).
RESULTS
Effect of addiction to sniffing lead-containing
substances on lead and calcium concentration levels: Table 4
and fig. 1 show that the mean lead concentration for sniffers was about
0.002107 mg/dl, while it was about 0.00189 mg/dl for control and the percentage of increment was estimated by about 11.6%.
The mean difference in lead concentration between sniffers and control subjects
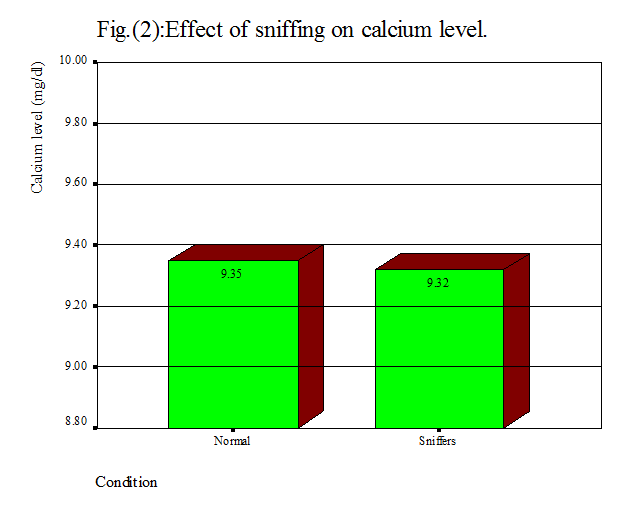
was not significantly different (P> o.o5). As well, table 4 and fig. 2 show
that the mean calcium concentration for sniffers was about 9.32 mg/dl, whereas
in control it was about 9.35 mg/dl, and
the percentage of increment was estimated by about 0.03%, which
statistically was insignificant.
Effect of addiction to sniffing
lead-containing substances on total protein concentration level: Tables 1
and fig.3 show that the level of total protein for sniffers was 6.78 g/dc,
corresponding to 6.75 g/dc for control and the percentage of increment was estimated by about 0.04%,
which was insignificant (P>0.05).
Effect of addiction to sniffing
lead-containing substances on albumin concentration level:
Statistical analysis showed that mean of albumin was significantly lower in
sniffers (4.04 g/dl) as compared to control subjects (4.19 g/dl).
The reduction in albumin level in sniffers as compared to control was estimated
by about 3.6% (Table 1 and fig.3).
Effect of addiction to sniffing
lead-containing substances on total bilirubin level concentration level: As shown
in table 1 and fig. 4 mean of total bilirubin in sniffers (0.69 mg/dl)
was significantly higher than that in control (o.63 mg/dl). The percentage of
increment in this parameter in sniffers as compared to control was about 9.5%.
Effect of addiction to sniffing
lead-containing substances on direct bilirubin level concentration level: The level
of direct bilirubin in sniffers (0.32 mg/dl) was significantly higher than that
reported by control (0.26 mg/dl). The difference between them was estimated by
about 18.8% (Table 1 and fig.4).
Effect of addiction to sniffing
lead-containing substances on indirect bilirubin concentration level: Table 1
and fig.3 show that sniffers had insignificant lower mean of indirect bilirubin
(6.43 mg/dl) as compared to the mean obtained by control (6.49 mg/dl). The
reduction in this parameter between sniffers and control subjects was estimated
by about 0.9%.
Effect of addiction to sniffing
lead-containing substances on AST concentration level: The mean
AST of sniffers was 20.47 mg/dl, while it was 20.95 mg/dl for control, with an
insignificant reduction estimated by about 2.3% (table 1 and fig. 3).
Effect of addiction to sniffing
lead-containing substances on ALT concentration level: Sniffers
reported 14.24 mg/dl level of ALT, whereas control recorded 14.13 mg/dl. The
difference between sniffers and control in this parameter was estimated by
about 0.8%, and it was insignificant (Table 1 and fig. 3).
Effect of addiction to sniffing lead-containing
substances on cholesterol concentration level: The mean level of
cholesterol was increased in sniffers (162.53 mg/dl) as compared to control
(158.65 mg/dl) by about 2.5% (Table 1 and fig. 5). This increment in level of
cholesterol under sniffers as compared to control subject was not significantly
different.
Effect of addiction to sniffing
lead-containing substances on triglyceride concentration level: In
contrast to cholesterol level, triglyceride in sniffers was decreased (40.74
mg/dl) as compared to that in control (42.08 mg/dl) (Table 1). The reduction in
this parameter in sniffers as compared to control was about 3.2%, which
statistically insignificant (Table 1 and fig. 5).
DISCUSSION:
With regard to blood lead level (Table 1 and
fig. 1) and calcium level (Table 1 and fig. 2), this study found that there was
no significant difference between sniffers cases (0.00211 mg/dl and 9.32 mg/dl)
and normal subjects (0.00189 mg/dl and 9.35 mg/dl, respectively). Higher levels of blood lead have been reported by a study
conducted in Sudanese towns by Abdalla et al. (2017), which found that
the poisoning of lead in exposed children was significantly higher at Medani
Town (0.0035 mg/dl), Khartoum City (0.00313 mg/dl), Atbara Town (0.00039
mg/dl), as compared to Eldeweam (0.00267 mg/dl) and Elobeid Towns (0.00250
mg/dl).
Whereas Saeed et al. (2017a) reported that mean blood lead concentration
in occupationally exposed workers in main Sudanese cities was found to be
0.0322 mg /dl, whereas in control was 0.0124 mg/dl. Generally, Needleman et
al. (1990), Bellinger et al. (1992) and Rogan et al. (2001)
mentioned that children biological susceptibility to lead is greater than that
of adults, because the developing human brain under goes rapid growth,
development and differentiation, and lead can interfere with these
extraordinary complex and delicate process. In the present study, age of
sniffers (9 – 18 years), duration of sniffers and the nature of the sniffed
substances may be behind the low levels of lead for sniffers as compared to
control. Higher quantities of calcium may increase the chance of binding sites
being occupied by calcium before lead is bound to them. Small quantities of
lead replace larger quantities of calcium used in activating key
neurotransmitters, notably protein kinase.
Accordingly, the ability of lead to replace calcium is believed to be a
probable cause of its ability to pass through and damage the blood / brain barriers.
The study also showed that most studied liver function parameters (Total
protein, indirect bilirubin, AST and ALT) in addition to lipid profile
parameters (cholesterol and triglyceride) were not significantly different as
comparing between sniffers and control subjects, but albumin level (Table 4 and
fig. 3) was significantly higher in sniffers as compared to control, while the
reverse was true for total and direct bilirubin (Table 4 and fig. 4). The liver
function values for control subjects were 6.75 mg/dl total protein, 4.19 mg/dl
albumin, 0.63 mg/dl total bilirubin, 0.26 mg/dl D. bilirubin, 4.49 mg/dl Ind.
bilirubin, 20.95 mg/dl AST and 14.13 mg/dl ALT, whereas for exposed cases were
found to be 6.78 mg/dl, 4.04 mg/dl, 0.69 mg/dl, 0.32 mg/dl, 6.43 mg/dl, 20.47
mg/dl and 14.24 mg/dl, respectively (Table 4). Lipid profile parameters for
control (cholesterol and triglyceride, fig. 5) reported 158.65 mg/dl and 42.08
mg/dl, respectively, whereas they were 162.53 mg/dl and 40.74 mg/dl for
sniffers, respectively. Similarly, concentrations of lead among the studied
sniffers, as shown in table 5, were not significantly affect both the liver
function and lipid profile parameters, except the total bilirubin. The slight
changes in some electrolyte and liver function (Ca++, total protein
and AST) and lipid profile (cholesterol) parameters that related to level of
lead in sniffers may be attributed to the effect of lead and other heavy metals
on the activity of enzymes that function in metabolism such as carbohydrate,
which eventually altered the levels of these parameters. Saeed et al.
(2017b) reported that there was a significant decrease in mean total protein
(from 7.210 g/dl to 5.354 g/dl), mean albumin (from 4.806 g/dl to 3.480 g/dl)
and mean globulin (from 2.404 g/dl to 1.862 g/dl) in control group in
comparison to lead exposed workers in major Sudanese cities, respectively. It
has been reported by Saeed et al. (2017c) that AST mean level in lead exposed workers in Sudanese
cities was found to be 26.9 U/L while in control the mean level was 16.1 U/L.
Whereas ALT mean level was increased from 18.3 U/L in control group to 28.6 U/L
in exposed group. Also Fowler (1981) and Flora (1991) stated that toxicity
of lead is closely related to its accumulation in certain tissues such as
hepatic tissues and however interferes with hepatic function and limited
ultra-structural changes Moreover, exposure to lead contamination result in
serious and undesired effects for human body, such as neurological behavioral
(Ycebilgic et al., 2003 and DE Marco, 2005), immunological (Bunn et
al., 2001), renal (Fowler et al., 1981,
Flora et al., 1991), hepatic (Flora et al.,
1991) and hematological dysfunction. Lawerence (1966) observed
that protein content was reduced with increasing of lead dose. The study also
demonstrated that, lead exposure may modify the metabolism of lipids, decrease
in the plasma membrane cholesterol and HDL cholesterol fractions. A significant
increase was observed in LDL in lead exposed workers (58.943 U/L) comparing to control (47.408 U/L) (Saeed et
al., 2017a). Furthermore, an
association between lead poisoning and renal diseases in humans has been
recognized and documented by several studies (Bernard et al., 1995).
Organic lead compound, which absorbed by ingestion or inhalation (such as
sniffers) are the most toxic substances. Regression analysis as shown in table
2, showed that there was a +ve and significant relationship between sniffers
lead levels and Ca++ (P≤0.05), albumin (P≤0.05), direct
bilirubin (P≤0.01), indirect bilirubin (P≤0.05) and triglyceride
(P≤0.01) levels, whereas the relationship between sniffers lead levels
and total protein, total bilirubin, AST, ALT and cholesterol was also +ve, but
insignificant. The positive relationship between lead levels and all there
parameters means that the studied sniffers have tendency to increase these
parameters by the B-values for each one unit increment in sniffers lead level,
for instance, for each one unit increment in exposed lead level, their Ca++.
This may eventually lead to alter the biological status of sniffers later on as
the result of changing expected in their enzymes.
CONCLUSION AND RECOMMENDATIONS:
It could be concluded that the blood lead and
calcium levels of both sniffers and normal control were within the normal
range, but lead level was slightly higher in sniffers than in control, while
calcium level was slightly reduced in sniffers than the control level. The
levels of albumin were significantly reduced in sniffers, whereas total and direct
bilirubin were significantly elevated in sniffers than in control, while other
liver function parameters (total protein, indirect bilirubin, AST and ALT)
showed no significant differences between sniffers and normal subjects. Lipid
profile parameters (cholesterol and triglyceride) showed no significant
differences between sniffers and control; however, the cholesterol was slightly higher in sniffers, whereas the reverse was
true for the triglyceride. Creating lead-free environment
through active involvement of all classes of society is of paramount importance
in order to elevate community awareness towards lead poisoning.
Acknowledgement
The authors would like to thank everyone who
contributed to complete this work.
REFERENCES:
Abdalla, F.A.B., Saeed, H.A.S., Abbas, A.A.
and Abdellah, A.M. (2017). Lead poisoning
from cars exhausts among primary school children in Sudan. Asian
Journal of Science and Technology, 08(10): 5970-5973.
Bellinger,
D. C., Stiles,
K. M.and Needleman,
H. L. (1992).
Low-level lead exposure, intelligence and academic achievement: A long-term
follow-up study.Pediatrics 90:855-861.
Bernard,
A. M., Vyskocil,
A.and Roels, H.
(1995). Renal effects in children living in the
vicinity of a lead smelter. Environ.
Res. 68:91-95.
Brady,
M. (1992). Heavy Metal: The social meaning of petrol sniffing in Australia.
Aboriginal Studies Press: Canberra.
Bunn,
T.L., Dietert,
R. R. and Ladics , G.S (2001).
Developmental munotoxicology assessment in the rat: Age, gender, and strain
comparisons after exposure to Lead. Toxicol Meth 11:41-58.
Butrimovitz,
1. G. P. and W. C pudy (1997).
(1977). The determination of lead in by Atomic Absorption Spectrometry.
Ann. Chim.
Blood plasma Acta 94.63 Pb (30).
Cannon,
D.C., Olifzky, I. and inkpen, J.A. 1974. Protein. Cli – chem. Principle and
technics,zed. Cannon and winkelman editors. New York, PP. 407 – 421.
Cheesbrough,
M. (2006) District laboratory practice in tropical countries, part 1, 2rd
addition, Cambridge University press, New York – USA, 350, 404 .
Chen
A, Dietrich KN, Ware JH, et al. 2005. IQ and blood lead from 2 to 7 years of
age: Are the effects in older children the residual of high blood lead
concentrations in 2-year-olds? Environ Health Perspect 113(5):597-601.
Cohen, Alan R.;
Trotzky, Margret S. and
Pincus, Diane (1981). Reassessment
of the Microcytic Anemia of Lead Poisoning". Pediatrics 67 (6):
904–906.
DE
Marco, M., R. Halpern and H. M. T. (2005). Barros,
Early behavioral effects of lead perintal exposure in rat pups, Toxicology,
211, 49-58.
Doumas,
B.T., Waston, W.A. and Bigg,h.G., 1971. Albumin standard and the measurement of
serum albumin with bromocresol green. Cli- chem. U.S. 31, 87 – 96.
Flora,
S. J.S.and
Tandon, S. K. (1991). Effect of combined exposure to lead and
ethanol on some biochemical indices in the rat.
Biochem Pharm 36:537-541.
Fowler,
B. A., Kimmel,
C. A. and Woods,
J. S. (1981). Chronic low-level lead toxicity in the
rat: III. An integrated assessment of long-term
toxicity with special reference to the kidney.
Toxicol Appl Pharmacol 56:59-77.
Grant,
L.D.and Davis,
J.M. (1989).
Effects of low-level lead exposure on paediatric neurobehavioural development:
current findings and future directions. In: Smith MA, Grant LD, Sors AI, eds.
Lead exposure and child development: an international assessment. Lancaster,
England, MTP Press, 49–118.
Hou,
Y.; Burkhard, B. & F. Müller (2013). Uncertainties in landscape analysis
and ecosystem service assessment. Journal of Environmental Management 127,
S117–S131
Lawerence, D. R. (1966).
Clinical Pharmacology (Third ed.). The London Bookworm (East Sussex, United Kingdom).
Needleman,
H. L., Schell,
A. and Bellinger, D. (1990).
The long-term effects of exposure to low doses of lead in childhood. An 11-year
follow-up report. N Engl J Med 322:83-88.
Reitman, S. and Frankel, M. 1957.
Acolorimetric method for the determination of serum glutamate oxaloacetatic
acid and pyruvic acid transaminases American of Clin-Pathol., 28: 56 –
63.
Rogan,
W. J., Dietrich,
K. N., Ware,
J. H. (2001).
The effect of chelation therapy with succimer on neuropsychological development
in children exposed to lead. N Engl J Med 344(19):1421-1426.
Saeed,
H.S.A., Abdellah, A.M., Abdalla, F.A.B. and Abbas,
A.A. (2017a). Effect
of air lead pollution on blood bilirubin and lactate dehydrogenase levels among
occupationally exposed workers in main Sudanese cities. IOSR Journal of Applied
Chemistry (IOSR-JAC) 10(1): 47-52
Saeed,
H.S.A., Abdellah, A.M., Abdalla, F.A.B. and Abbas,
A.A. (2017b). Biochemical
Effects of Lead Toxicity on Serum Total Protein, Albumin and Globulin Levels in
Occupationally Exposed Workers in Major Sudanese Cities. International Journal
of Emerging Technology and Advanced Engineering Website 7(3): 132-138.
Saeed,
H.S.A., Abdellah, A.M., Abdalla, F.A.B. and Abbas,
A.A. (2017c). Effect
of lead exposure on biochemical tests in blood of occupationally exposed
workers in major Sudanese cities. International
Journal of Development Research, 7(5): 12694-12699
Ycebilgic,
G., R., Bilgin, L. and Tamer, S. (2003). Tukel, effect of lead on Na-K ATP ase and Ca2+. ATPase
activities and lipid peroxidation in blood of worker Intj.Toxicol, 22,
95-97.
Zheny
XM, Resnick RJ, Shalloway D (2008) Apoptosis of estrogen-receptor negative
breast cancer and colon cancer cell lines by PTPα and Src RNAi. Int J
Cancer 122: 1999–2007.