|
Greener Journal of Human Physiology and Anatomy
Vol. 3(1), pp. 01-10, 2021
ISSN: 2354-2314
Copyright ©2021, the copyright of this article is
retained by the author(s)
https://gjournals.org/GJHPA
|

|
Increased Heart Rate and Steady State
Among Sickle Cell Patients Seen in Sokoto, North-West Nigeria
1Michael I.
Ikedue; 2Frank B.O. Mojiminiyi; 3Muhammad A. Ndakotsu; 4Simeon A. Isezuo;
5Adamu J. Bamaiyi
1MSc, Department of
Physiology. Usmanu Danfodiyo University, Sokoto, Nigeria.
2PhD,
Professor, Department of Physiology, Usmanu Danfodiyo University, Sokoto,
Nigeria.
3MBBS, FMCPath,
Associate Professor of Haematology and Consultant Haematologist Haematology
Department, Usmanu Danfodiyo
University, Sokoto, Nigeria.
4MBBS, FMCP, Professor of Medicine and Consultant
Cardiologist, Internal Medicine Department, Usmanu Danfodiyo University, Sokoto,
Nigeria.
5MBBS, PhD, Senior
Lecturer, Department of Physiology, Usmanu Danfodiyo University, Sokoto,
Nigeria.
|
ARTICLE INFO
|
ABSTRACT
|
|
Article No.: 071021064
Type: Research
|
Background: Sickle cell
anaemia (SCA) remains a major cause of morbidity and mortality among patients
homozygous for the traits. However, some patients homozygous for the disease
do not have vaso-occlusive crisis as much as others, and the reason for this is not yet clear.
Objectives: The present study
aimed to assess haemodynamic and haematological parameters among adult SCA
patients (with crisis or in steady state) attending clinics and compared same
with normal adults.
Methods: One hundred and six consenting consecutive
subjects were recruited under the groups; Steady (n = 53) or Crisis (n = 53)
and compared the results with 40 apparently healthy genotype AA adults.
Results: We report that, the steady group, has
significantly less Packed cell volume (PCV) compared to the normal group
(27.39 ±4.36% Vs 33.44 ±1.80%, p <0.00005), but
showed higher values of PCV compared to the crisis group (27.39 ±4.36% Vs 25.67 ± 4.23%, p <0.0422). The steady group
compared to the crisis group, also demonstrated enhanced heart rate (HR)
(84.19 ± 16.53 bpm Vs
78.96 ± 15.90 bpm, p = 0.019) and longer QTc (429.00 ± 37. 33 Vs 416.15
±35.10, p = 0.0060).
Conclusions:
Enhanced HR, rate pressure product (RPP) and optimum systolic phase of
the cardiac cycle (QTc) in the steady state sub-group, may result in better ventricular functions and
cardiac output. Therefore, whole blood or intravenous fluid infusion in SCA
patients may improve the chronotropic and inotropic
properties of the heart, enhance tissue perfusion and oxygen delivery that
will help reduce crisis in the patients.
|
|
Accepted: 15/07/2021
Published: 31/07/2021
|
|
*Corresponding Author
Dr Adamu
Jibril Bamaiyi
E-mail: adamu,jibril @udusok.edu,ng; abamaiyi@ yahoo.com
Phone: +2348030925695
|
|
Keywords: Sickle
cell anaemia; Homozygous trait; Vaso-occlusive
crisis; Steady state; Heart rate; Whole blood; Intravenous infusion; Cardiac
output; Inotropic; Chronotropic
|
|
|
|
ABBREVIATIONS
BMI = Body Mass Index, SBP=
Systolic Blood Pressure, PP= Pulse Pressure, HR = Heart Rate, PRP
= pressure reference point, HbSS = Sickled cell haemoglobin, SO2
= Percentage Oxygen Saturation, PCV = Packed Cell Volume, VOC = Vaso-occlusive crisis,
P-R = P-R interval, QT = QT interval, QTc=
QT corrected for heart rate, ISC = Irreversible sickled
cell, RPP = Rate pressure product, SCA = Sickle cell anaemia, ECG = Electrocardiogram, UDUTH = Usmanu Danfodiyo
University Teaching Hospital, AHA = American Heart Association, LAE
= Left atrial enlargement, RAE = Right atrial enlargement, LVH = Left
ventricular hypertrophy, RVH = Right ventricular hypertrophy, BBB
= Bundle branch block, Na-EDTA
= Sodium - ethylene diaminetetra acetic acid, HIV= Human immunodeficiency virus, Kg
= Kilogramme, cm = Centimetre,
m2 = squared metres,
INTRODUCTION
Sickle cell anaemia
(SCA) is still the largest hereditary haemoglobinopathy
in the world (1, 2). With Africa,
especially Nigeria having the highest burden globally (2-4). And in terms of mortality, about 10% - 14% of the patients
do not live beyond 20 years (5) and by
the fifth decade of life about half of their population might have died (5). However, not all SCA patients come down
with complications or crisis, even though there is no any preferential
treatment given to them. Indeed, there is still ambiguity as to the factors
that are responsible for this polymorphism among SCA patients (6). In this regard, the electrocardiography of
SCA patients have been documented, and some cardiovascular abnormalities in SCA
have been reported (5-7). Nevertheless,
data is almost non-existent on the haemodynamic
features in the SCA category of patients with crisis compared with those
without crisis (6, 8).
Hypoxia is also a
pathophysiological feature of vaso-occlusive crisis (VOC) in SCA patients (1). Therefore, the rate and volume of blood
delivered to the tissue may explain the VOC tendencies in the patients. In the
presence of optimum systolic functions, increased HR will result in improved
cardiac output and oxygen delivery to tissues will be better. In the
present study therefore, we assess in addition to the haematological
parameters, some tissues oxygen delivery indices and haemodynamic
parameters including blood pressure, heart rate and electrocardiographic
features in the patients with crisis, using pulse oximeter
and electrocardiograph respectively. The results were compared with those who
do not have crisis (steady state) and compared same with normal AA genotype
group. The haemodynamic factors that might be
conferring crisis-free advantage on the steady state patients, but not the
other group were noted.
Subjects, Materials and Methods
Ethical approval with
reference UDUTH/HREC/2016/No.428 for the study was obtained from the Ethical
Committee of the Usmanu Danfodiyo
University Teaching Hospital (UDUTH), Sokoto.
Informed consent was obtained from all participants. And the research complied with
regulations concerning human research, as enshrined in the Helsinki declaration
(9).
It was a cross
sectional study that assessed the ECG patterns, blood pressure and some blood
parameters of adult SCA patients. This
study was carried out in the Hematology outpatient clinic of (UDUTH), Sokoto, North-Western
Nigeria. A total of 106 sickle
cell anemia (SCA) patients of both sexes were recruited, comprising; a group
with crisis (n=53), the group with steady state (n=53), and they were compared
with 40 normal (genotype AA) subjects sourced from the hospital environment. The normal subjects were also selected
randomly among medical and nursing students, hospital workers and members of
the local community who served as controls.
Inclusion
criteria
Only SCA patients
older than 18 years, confirmed by HbSS genotype by Hb electrophoresis were included in the study. Apparently
healthy adults from the same environment were recruited as the normal group.
Exclusion
criteria
Excluded in the study
sample are, patients with reported leukemia, renal disease, HIV infection, congenital or acquired heart disease,
pregnancy, severe anaemia, excessive use of alcohol
(more than 16 g daily) (10), and
significant use of tobacco.
Definitions
Crisis sub-group:
Individuals homozygous for the SCA traits, who had vaso-occlusive
crisis (VOC) within the last 4 weeks (11)
Steady sub-group:
Individuals homozygous for the SCA traits but had no VOC in the last 4 weeks (11)
Normal group:
Apparently healthy individuals who are homozygous for AA genotype
Anthropometric
measurements
The weights
(Kg) and heights (cm) of participants were measured by standard procedures and
the body mass index (BMI, kg/m2) derived from the corresponding
weights and heights.
Blood
Pressures
Standard
procedures were followed in measuring brachial blood pressure, using Accuson mercury sphygmomanometer with an appropriate cuff
on the left or right arm. The average of five readings were taken as the
Systolic and diastolic blood pressures, respectively at 1st and 5th
Korotkoff sounds, in sitting position after 5 minutes
of rest with the arm at pressure reference point (PRP) and readings taken to
the nearest 2mmHg (12).
Radial pulse rate
was assessed using standard procedures.
Electrocardiography
Dr. Lee model
electrocardiography machine (made in China) at a paper speed of 25mm/s and
standardized at 0.1mv/mm was used. The recommendation of the American Heart
Association (AHA) (12), were adopted
using the standard resting 12 lead ECG. Atrial and
ventricular rates, rhythm, P-wave, P-R interval, QRS duration, abnormality and
axis in frontal plane directed to the region between -300 to 1050
were taken as normal axis. Similarly, QT and QTc
intervals were retrieved and recorded. Right
atrial enlargement (RAE), defined as the presence of peaked P-wave with
amplitude of 2.5mm or more in lead II, III and avF or
as P-pulmonale were sought
for. Left atrial enlargement (LAE), defined as the presence
of notched P with duration of 0.12 seconds or more or defined as P-terminal force in
V1 equal or more negative than 0.04 mm.sec or as P-mitrale were sought for. Biatrial
enlargement was considered when both RAE and LAE parameters occurred together
in same patient. The presence of Left ventricular hypertrophy (LVH) were defined as per Sokolow-lyon
criteria (13) as S-wave in V1 + R-wave in
V5 or V6 > 35mm or as defined by Araoye criteria
S-wave in V2 + R-wave in V5 or V6 >35mm in female or > 40mm in male (13). But, after the presence of bundle branch
blocks (BBB) were excluded (14, 15). Right ventricular hypertrophy (RVH) was
defined as per dominant R-wave (a) R wave in V1 ≥ 7mm (b) R/S ratio
≥1 in V1 or alternatively R/S ratio in V5 or V6 <1 (c) R wave in V1+S
wave in V5 or V6 > 10.5mm (d) qR complex in V1.
Combined ventricular hypertrophy defined as meeting the criteria for both LVH
and RVH.
Determination of Packed Cell Volume (PCV)
Each
subject’s venous blood was collected by venipucture
as described by (16). Three millilitres (mls) of blood was
drawn from a peripheral vein of either upper limb because of their prominence
and accessibility using 5 mls syringe and 21G needles
(17) and transferred into Sodium -
ethylene diaminetetra acetic acid (Na-EDTA)
tubes. The PCV was determined using microhaematocrit method. Two-third of the capillary tube
was filled with blood sealed at one end using plasticine,
then placed appropriately in the microheamatocrit
centrifuge and allowed to revolve at 10000 rev/min for
5 minutes. Subsequently, the tube was placed on a microhaematocrit
reader for proper PCV estimation.
Determination
of percentage of irreversibly sickled cells (ISCs)
A thin
blood film was made using wedge technique, allowed to air-dry and labeled. The
film was flooded with Leishman stain and allowed for
2 minutes, then diluted with equal volume of buffered distilled water and left
for about 8 minutes, after which the stain was washed off completely with
distilled water. Following other standard procedures, the percentages of
irreversibly sickled cells were calculated by counting 100 cells in various
fields on the slide with normal and sickled cells counted. The percentage of
ISCs was calculated using the following formula;
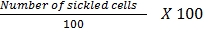
Determination
of percentage of oxygen saturation
Oxygen
saturation was determined using the pulse oximeter
(PC – 60B1, USA) by appropriately placing the participant’s index finger in the
sensor of the oximeter (18, 19) and the
readings were recorded.
Statistical
analysis
Database storage and analysis was done
using IBM SPSS (version 23.0) package. Exploratory data analysis was
performed to detect incorrect entries and normality was examined using Shapiro-Wilk test. Data were presented as mean ± standard
deviations (mean ± SD) for continuous variables and tables were used in
presenting data. Multiple regression analysis was used to determine
β-coefficients for confounding factors and confidence intervals (CI) of
quantitative variables. Where linear relationships are sought between two
factors, the slope of the relations using the equation for linear regression,
with the corresponding squared-R determined, using Microsoft excel software.
Single factor ANOVA were also used in assessing the difference in mean among
the groups, also using Microsoft excel software. The level of statistical
significance (α) for the test was set at P<0.05
RESULTS
The mean ages of the groups; Normal,
Steady and Crisis were respectively 21.38±2.31, 20.92±2.71, 21.47±2.80 and were
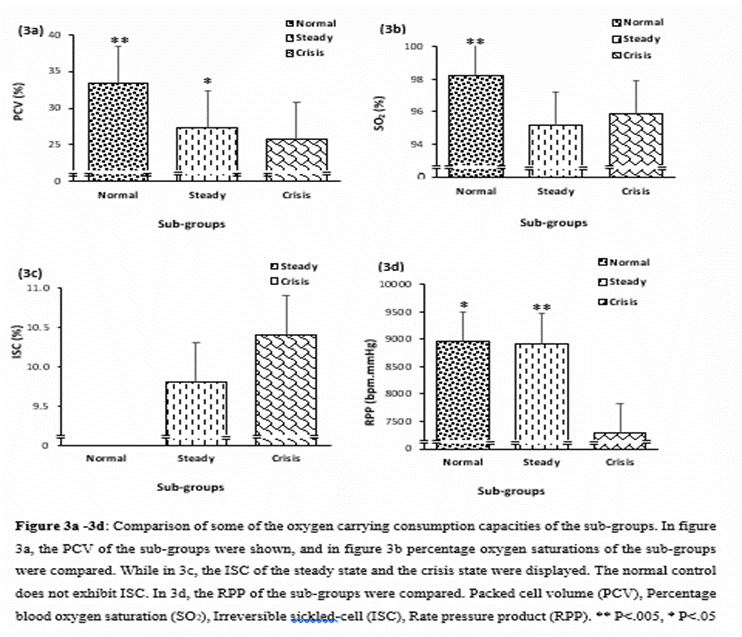
not significantly different (p=0.6907). However, the normal group, compared to
the steady and crisis groups, had higher PCV (33.44 ±1.80, Vs
27.39 ±4.36 and 25.67 ±4.23, P<0.0001) and SO2(98.20
±0.88 Vs 95.15 ±3.25 and 95.89 ±3.55, P<0.0001).
Refer figures 3a and 3b. But the normal group had no irreversibly sickled cells
(ISC) compared to the SCA steady and crisis groups, 9.76±4.22 and 10.37±5.01,
respectively. See figure 3c.
Haemodynamic values of
the study participants
The normal group had
higher SBP compared to the SCA groups (122.9 ±10.4 mmHg Vs
106.8 ±12.23 mmHg, P<0.00005). The pulsatile component of the blood, pulse
pressure (PP) was similarly higher in the normal group as compared to the SCA
groups (49.2 ±1.1 mmHg Vs 42.6 ±13.5 mmHg,
P<0.05). However, there was no any significant differences
in both the SBP and PP between the SCA sub-groups. See table 1. But, the HR
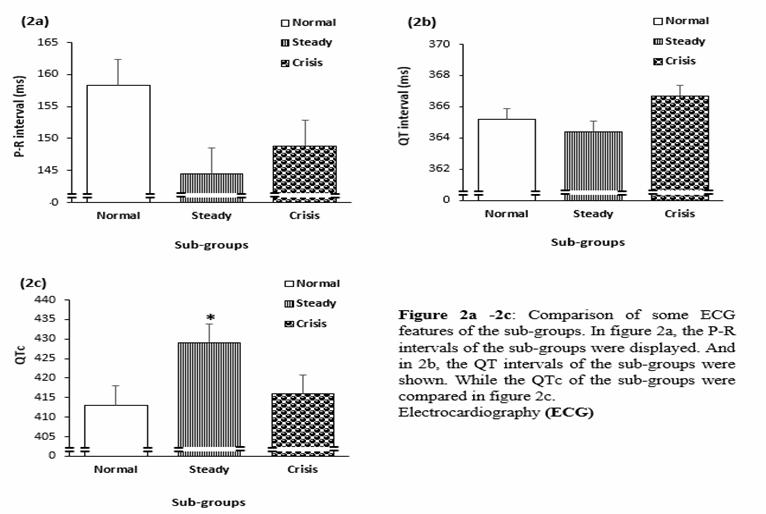
among the SCA sub-group in steady state is enhanced, compared to both the
normal and the SCA sub-group that was in crisis state (84.2 ±16.5 bpm Vs 77.1 ±14.3 bpm, P=0.0050). Refer table 1. The corrected QT, (QTc) which represent the ventricular depolarization and
repolarization of the electrical functions of the heart was also significantly
higher among the steady sub-group, compared to the other groups (429.0 ±37.3ms Vs 411.3 ±38.2 ms, P=0.018). See
figure 2c. There was however, no statistically significant differences among
the groups, in the P-R interval, which represent the period of atrial
depolarization among the groups (P = 0.1078). See figure 2a.
.
Table 1: Characteristics of the study populations
|
Parameter
|
Normal (n=40)
|
Steady (n=53)
|
Crisis (n=53)
|
|
Age ±SD (yrs)
|
21.4
±2.3
|
20.9
±2.7
|
21.5
±2.8
|
|
BMI ±SD (Kg/M2)
|
24.3
±0.6***
|
19.3
±3.4
|
19.9
±3.2
|
|
Height ±SD (cm)
|
158.4
±7.0
|
160.5
±8.8
|
161.0
±9.3
|
|
SBP ±SD (mmHg)
|
122.9
±10.4***
|
108.1
±11.8
|
105.5
±12.6
|
|
PP ±SD (mmHg)
|
49.2
±1.1*
|
44.2
±13.8
|
41.1
±13.1
|
|
HR ±SD (bpm)
|
76.5
±12.9
|
84.2
±16.5*
|
79.0
±15.9
|
***
p<0.00005. BMI = Body
Mass Index
Ventricular chamber and walls characteristics
of the study populations
The cardiac chamber
or myocardial walls might be within normal ranges, as can be deduced from the
ECG precordial results presented in table 2. And the results were not
significantly different among the three groups.
Table 2: Precordial ECG results of the study
groups
|
|
Normal (n =40)
|
Steady (n =53)
|
Crisis (n =53)
|
|
SV1 (mm)
|
9.9 ± 5.0
|
9.6 ±4.9
|
7.8 ±4.6
|
|
RV6 (mm)
|
10.4 ±5.0
|
11.7 ±6.9
|
13.6 ±6.2
|
|
SV1 +RV6 (mm)
|
20.3 ±8.4
|
21.4 ±10.5
|
21.4 ±8.8
|
|
RV1 (mm)
|
2.5 ±1.8
|
2.2 ±1.7
|
2.2 ±1.8
|
|
RV1/SV1
|
0.3 ±0.2
|
0.3 ±0.3
|
0.3 ±0.3
|
Oxygen carrying capacities and the RPP of the
study populations
The normal group of
the study population has a significantly higher PCV (P<0.0001), compared to
the SCA groups (see figure 3a). Furthermore, the steady sub-group also has a
significantly higher PCV compared to the sub-group described with crisis (P =
0.0422).
Although, all the
sub-groups had good RPP values (8954.0 ±2828.8, 8924.2 ±2228.5 and 7282.2
±3381.8 for the normal, steady and crisis sub-groups, respectively), the
sub-group in crisis had significantly lower RPP compared to both the sub-group
in steady state and the normal controls (F =5.777, Fcir
=3.058, P =.0039). See figure 3c.
Moreover, in the
normal group, only PCV showed significant correlation with the blood level of
SO2 (β-coeff = 0.353, CI = 0.007 – 0.385, p = 0.041. In the steady
sub-group of SCA however, HR, BMI and PCV showed significant positive
correlation with SO2 (P < 0.05). On the other hand, none of the
variables in the crisis sub-group of SCA showed significant relationship with
blood SO2 levels (refer table 3). Indeed, the steady sub-group had
significantly higher HR (84.2 ±16.5 bpm Vs 79.0 ±15.0 bpm, P =0.0270) and
PCV (27.4 ±4.4 % Vs 25.7 ±4.2%, P =0.0422) compared
to the crisis sub-group. See figure 3a. Furthermore, although the steady
sub-group also showed correlations of the BMI with the SO2 levels
(see table3), there is no statistically significant differences in the BMIs of
the Steady or crisis sub-groups of the SCA populations of the study (refer
table 1) and so unlikely to confer any comparative advantage on the sub-group
in steady state. Moreover, the present data showed that at significantly low
PCV, HR bears a negative relationship with SO2. See table 3
The HR was positively
associated with QTc among all the three sub-groups
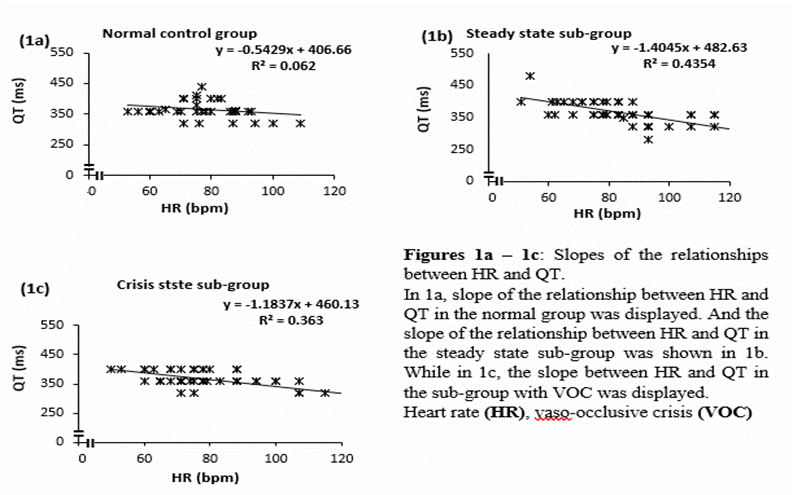
studied. See table 4 Indeed, QT (unadjusted for HR) is inversely proportional
to the HR (6,20,21). However, this may not be the case
when the changes in HR is within physiological
reference range for adult humans (60 – 100 bpm). Such
case is as found in the present data, where the QT intervals, independent of HR
(QT/HR) of the study populations were respectively 4.9 ±0.9, 4.5 ±1.4
and 4.4 ±1.8 (F =1.595, Fcrit =3.0603, P =0.209) for
the normal, steady and crisis groups and it is in tandem with the slopes of the
relationships between HR and QT (figure 1a – 1c). Furthermore, table 4 above
showed that, at significantly low PCV, negative correlations exist between PCV
and QTc. And this is significantly so, with further
escalation in the PCV value, as shown in the crisis sub-group
(β-coefficient = -0.394, CI =-5.955 - -0.431, P = 0.025). Otherwise, none
of the other variables showed significant correlations with the QTc (P > 0.05).
Table 3: Correlations (standardized
β-coefficients) of the haemodynamic parameters
with patients’ level of arterial haemoglobin oxygen
saturation, SO2
|
|
Normal (n =40)
|
Steady (n = 53)
|
Crisis (n = 53)
|
|
|
β-coeff
|
CI
|
P-Value
|
β-coeff
|
CI
|
P-Value
|
β-coeff
|
CI
|
P-Value
|
|
HR
|
0.053
|
-0.021 – 0.029
|
0.768
|
0.359
|
0.13-0.115
|
0.016
|
-0.039
|
-0.083 – 0.065
|
0.803
|
|
SBP
|
0.032
|
-0.030 – 0.036
|
0.869
|
-0.125
|
-0.114–0.049
|
0.427
|
-0.089
|
-0.142 – 0.089
|
0.645
|
|
PP
|
-0.043
|
-0.056 – 0.044
|
0.817
|
-0.019
|
-0.083–0.044
|
0.543
|
0.063
|
-0.090 – 0.126
|
0.734
|
|
BMI
|
0.019
|
-0.073 – 0.082
|
0.912
|
0.324
|
0.027-0.534
|
0.031
|
-0.122
|
-0.874 - 0.273
|
0.477
|
|
PCV
|
0.353
|
0.007 – 0.385
|
0.041
|
0.381
|
0.069-0.448
|
0.009
|
0.171
|
-0.193 - 0.485
|
0.390
|
|
ISC
|
-
|
-
|
-
|
-0.229
|
-0.353– 0.031
|
0.099
|
-0.143
|
-0.387 - 0.178
|
0.459
|
HR = Heart Rate (bpm), SBP= Systolic Blood Pressure (mmHg), PP=
Pulse Pressure (mmHg), BMI= Body Mass Index (Kg/m2), PCV
= Packed Cell Volume (%), ISC = Irreversible Sickle Cell (%).
Table 4: Correlations (standardized
β-coefficients) of the hemodynamic parameters with patients corrected
electrical systolic period, QTc
|
|
Normal (n =40)
|
Steady (n = 53)
|
Crisis (n = 53)
|
|
|
β-coeff
|
CI
|
P-Value
|
β-coeff
|
CI
|
P-Value
|
β-coeff
|
CI
|
P-Value
|
|
HR
|
0.595
|
0.910 –
2.818
|
0.000
|
0.369
|
0.116 –
1.551
|
0.024
|
0.417
|
0.320 –
1.520
|
0.004
|
|
SBP
|
-0.045
|
-1.431 – 1.080
|
0.778
|
-0.007
|
-1.163 – 1.119
|
0.969
|
-0.103
|
-1.234 – 0.650
|
0.535
|
|
PP
|
-0.195
|
-3.057 –
0.731
|
0.220
|
0.089
|
-0.650
-1.130
|
0.589
|
0.108
|
-0.579
-1.177
|
0.496
|
|
BMI
|
-0.060
|
-3.539 – 2.343
|
0.681
|
0.030
|
-3.224 – 3.877
|
0.854
|
-0.084
|
-4.438 – 2.465
|
0.567
|
|
PCV
|
0.040
|
-5.351 –
7.107
|
0.776
|
-0.007
|
-2.707 –
2.592
|
0.965
|
-0.394
|
-5.955 -
-0.431
|
0.025
|
|
ISC
|
-
|
-
|
-
|
-0.019
|
-2.864 -2.515
|
0.897
|
-0.207
|
-3.737 – 0.858
|
0.213
|
Heart Rate (HR), Systolic Blood
Pressure (SBP), Pulse Pressure (PP), Body Mass Index (BMI),
Packed Cell Volume (PCV), Irreversible Sickle Cell (ISC)
P -R Intervals
The P-R interval in
the normal group showed no significant correlations with any of the variables
determined (p >0.05). However, the sub-group in steady state showed negative
significant correlations with the HR, but a positive relationship with ICS
(β-coeff = -0.370, CI = -1.548 - -0.227, P =
0.010 and β-coeff = 0.352, CI = 0.864 – 5.818, P
= 0.026, respectively). As found with the steady sub-group, the crisis
sub-group showed significant negative correlation between the P-R interval and
HR (β-coeff = -0.361, CI = -1.378 - -0.106, P
=0.023). See table 5. But other variables showed no significant correlation
with the P-R interval. P-R interval have some
relationship with haemodynamic functions (22), but
may only apply where there is chronic insult to the cardiovascular system (23).
Table 5: Correlations (standardized
β-coefficients) of the haemodynamic parameters
with patients’ electrical diastolic period, P-R interval
|
|
Normal (n =40)
|
Steady (n = 53)
|
Crisis (n = 53)
|
|
|
β-coeff
|
CI
|
P-Value
|
β-coeff
|
CI
|
P-Value
|
β-coeff
|
CI
|
P-Value
|
|
HR
|
-0.130
|
-1.133 –
0.532
|
0.468
|
-0.370
|
-1.548 - -0.227
|
0.010
|
-0.361
|
-1.378 -
-0.106
|
0.023
|
|
SBP
|
0.046
|
-0.964 – 1.228
|
0.808
|
-0.036
|
-1.177 – 0.924
|
0.809
|
0.103
|
-0.735 – 1.281
|
0.587
|
|
PP
|
0.188
|
-0.823 –
2.484
|
0.314
|
0.201
|
-0.242 –
1.397
|
0.163
|
-0.148
|
-1.335 –
0.563
|
0.416
|
|
BMI
|
0.317
|
-0.213 – 4.921
|
0.071
|
-0.069
|
-4.071 -2.469
|
0.624
|
-0.233
|
-6.217 – 1.118
|
0.168
|
|
PCV
|
-0.077
|
-6.693 –
4.181
|
0.641
|
0.162
|
-0.960
-3.920
|
0.228
|
-0.025
|
-3.107
-2.731
|
0.897
|
|
ISC
|
-
|
-
|
-
|
0.352
|
0.864 – 5.818
|
0.009
|
0.026
|
-2.354 – 2.697
|
0.892
|
|
|
|
|
|
|
|
|
|
|
|
Heart Rate (HR), Systolic Blood
Pressure (SBP), Pulse Pressure (PP), Body Mass Index (BMI),
Packed Cell Volume (PCV), Irreversible Sickle Cell (ISC)
DISCUSSION
The main findings in
the present study are; SCA patients have significantly lower BMI, systolic
blood pressure, pulse pressure and PCV compared to the normal individuals. The
normal group also showed better blood oxygen saturation compared to the SCA
groups. Indeed, the steady sub-group of the SCA populations have
higher PCV level compared to the crisis sub-group. Furthermore, the steady
sub-group of SCA had significantly higher HR compared to both crisis sub-group
and the normal group. Although, both sub-groups of the SCA had increased HR,
the steady sub-group had significantly enhanced HR. In this regard, the steady
sub-group of SCA showed significant relationship between the HR and SO2,
but this was not seen in the crisis sub-group. Indeed, the normal group, which
had much lower HR showed no significant relationship between the HR and SO2.
Moreover, the sub-group in steady state showed significant relationship between
PCV and SO2, similar to what obtains in the normal population
sampled. However, the sub-group with crisis displayed not any appreciable
relationship between the PCV and SO2. The normal and steady state
sub-groups also demonstrated significantly higher heart rate-pressure product
(RPP) compared to the sub-group with crisis. Furthermore, all the three sub-groups
demonstrated significant relationship between its HR and corrected QT (QTc). There was also no evidence of ventricular chamber
abnormalities (see table 2).
Heart rate and QTc
Although QT intervals
beyond 429 ms is reported to be associated with
all-cause mortality among cardiovascular disease patients (not SCA) (20, 21), a study (6) indicated that most SCA patients may be having borderline or
prolonged QT interval without clinical symptoms. Nevertheless, cardiovascular
adjustments require that in anemia, typical of SCA patients (24, 25), that there will be upregulation of the HR to improve cardiac output (CO) in
order to meet the tissue needs (26). Increased HR is an important component of the
CO which is compensatorily improved according to the
body need (27-29). However, a study, (30) observed that substantially high HR is
associated with SCA crisis. The above study was not able to establish this
relationship with the pre-crisis HR of the SCA patients, in which case the
crisis may be the cause of the raised HR.
Furthermore, the studies that reported the untoward influence of HR on
cardiovascular outcomes didn’t establish this in the general population or SCA
patients (31), rather they were carried
out among subjects suffering from ardiovascular
diseases (30, 32, 33). Moreover, the earlier studies (34, 35) worked on resting HR to determine the
effects on all-cause mortality, but not in compensatory instances like, chronic
mild anaemic adaptation. The increased HR may be
compensatory in chronic mild haemolytic conditions (26). Moreover, a prospective community-based
study comprising over twenty thousand subjects reported that cardiac deaths are
more associated with lower HRs than moderately higher HRs (36). Consequently, the present study report
that both HR and QTc (a better index of
cardiovascular risk than QT) are significantly higher in the steady sub-group
compared to the crisis sub-group, because of the better compensatory tendencies
of the former sub-group, hence is able to stay without much crisis. This is
corroborated by the RPP values of the sub-groups displayed in figure 3c.
Meanwhile, as seen in
the present data, haemodynamic parameters
does not bear any relationship with P-R interval in individuals without
cardiovascular perturbations (22, 23).
PCV and SO2
Higher PCV levels,
enhanced HR and RPP demonstrated by the sub-group in steady may explain the
better oxygen delivery and utilization to the tissues and less crisis among
individuals who might be homozygous for the SCA genotype. Importantly, the
present study showed that this is true, even when the PCV is significantly
lower than the average value observed among the normal population. Indeed, SCA
patients have lower PCV levels (24, 25).
Furthermore, hypoxia is a pathophysiological
feature of vaso-occlusive crisis (VOC) in SCA
patients (1). The rate and volume of
blood delivered to the tissue may explain the VOC tendencies or otherwise in
SCA patients. Moreover, increase sympathetic discharge is said to aggravate SCA
crisis (8), but intravenous fluid
infusion is recommended in individuals with crisis (37, 38). Indeed, plasma
volume expansion in the patients will enhance venous return and likely to
increase heart rate by the Bainbridge mechanism (28,
29). Furthermore, repeated blood transfusion is reported to be able to
reduce the risk of stroke in SCA patients by 92% (6). Importantly, in the presence of optimum systolic functions,
increased HR will result in improved cardiac output and oxygen delivery to
tissues will be better. Consequently, the present study showed significant
relationship between HR and SO2 in the steady sub-group.
Therefore, as much as fluid therapy is
indicated in alleviating crisis in SCA patients, the PCV must be optimum
alongside normal cardiac functions, to achieve steady state. Otherwise, safe
whole blood transfusion will be a better option, if available. Indeed, blood
transfusion will provide both plasma volume expansion and PCV enhancement.
Limitations of study
The study sample size
is small and may not be representative of the larger population of SCA patients
in this environment. The study is also limited by its cross-sectional nature,
therefore couldn’t proclaim causal effect.
Proper measurement of the myocardiac systolic
function would have been worthwhile, but we had no access to echocardiographic
machines when collecting the data and therefore couldn’t carry out the
measurements of ejection fraction or cardiac output.
CONCLUSION
Africa and indeed
Nigeria has the highest burden of sickle cell disease globally (2-4), on a background escalating economic
hardship. Consequently, we recommend
that in addition to enhancement of factors such as foetal
hemoglobin induction, reductions of cell elements aggregations, or increased
nitric oxide bioavailability, SCA patients with optimum PCV alongside enhanced
or normal ventricular functions (see table 2 and figure 3c) have the advantage of
overcoming some of the hypoxic tendencies, and are likely to experience less
crisis.
Acknowledgement
We are thankful to
our patients for consenting to participate in the study. We are also grateful
to Drs Isah Oboirien and Muawiyya Usman, of the Department of Internal medicine for the
technical assistance on ECG interpretations. Mr. & Mrs. Igbokwe
are sincerely appreciated for the assistance in initial data organization and
normal subjects sourcing, respectively.
Duality of interest
None declared
Source of funding
No funding was
received for this research
REFERENCES
1.
Chirico EN, Faës C, Connes P, Canet-Soulas E,
Martin C, Pialoux V. Role of Exercise-Induced Oxidative Stress in Sickle Cell
Trait and Disease. Sports Med. 2016;46(5):629-39.
2.
Adeyemo T, Ojewunmi O, Oyetunji A. Evaluation
of high performance liquid chromatography (HPLC) pattern and prevalence of
beta-thalassaemia trait among sickle cell disease patients in Lagos, Nigeria.
The Pan African medical journal. 2014;18:71-.
3.
Aliyu ZY, Kato GJ, Taylor Jt, Babadoko A,
Mamman AI, Gordeuk VR, et al. Sickle cell disease and pulmonary hypertension in
Africa: a global perspective and review of epidemiology, pathophysiology, and
management. Am J Hematol. 2008;83(1):63-70.
4.
Makani J, Williams TN, Marsh K. Sickle cell
disease in Africa: burden and research priorities. Ann Trop Med Parasitol.
2007;101(1):3-14.
5.
Platt OS, Brambilla DJ, Rosse WF, Milner PF,
Castro O, Steinberg MH, et al. Mortality in sickle cell disease. Life
expectancy and risk factors for early death. The New England journal of
medicine. 1994;330(23):1639-44.
6.
Mueller BU, Martin KJ, Dreyer W, Bezold LI,
Mahoney DH. Prolonged QT interval in pediatric sickle cell disease. Pediatr
Blood Cancer. 2006;47(6):831-3.
7.
Lamers L, Ensing G, Pignatelli R, Goldberg C,
Bezold L, Ayres N, et al. Evaluation of left ventricular systolic function in
pediatric sickle cell anemia patients using the end-systolic wall
stress-velocity of circumferential fiber shortening relationship. Journal of
the American College of Cardiology. 2006;47(11):2283-8.
8.
Manwani D, Frenette PS. Vaso-occlusion in
sickle cell disease: pathophysiology and novel targeted therapies. Blood.
2013;122(24):3892-8.
9.
World Medical A. World Medical Association
Declaration of Helsinki: ethical principles for medical research involving
human subjects. Jama. 2013;310(20):2191-4.
10.
Kumar P, Clark M. In: Kumar P, Clark M,
editors. Kumar and Clark's Clinical Medicine: Elsevier; 2016.
11.
Ballas SK, Smith ED. Red blood cell changes
during the evolution of the sickle cell painful crisis. Blood.
1992;79(8):2154-63.
12.
Perloff D, Grim C, Flack J, Frohlich ED, Hill
M, McDonald M, et al. Human blood pressure determination by sphygmomanometry.
Circulation. 1993;88(5 Pt 1):2460-70.
13.
Sokolow M, Lyon TP. The ventricular complex
in left ventricular hypertrophy as obtained by unipolar precordial and limb
leads. 1949. Ann Noninvasive Electrocardiol. 2001;6(4):343-68.
14.
Ogunlade O, Akintomide AO. Assessment of
voltage criteria for left ventricular hypertrophy in adult hypertensives in
south-western Nigeria. J Cardiovasc Dis Res. 2013;4(1):44-6.
15.
Azeem T, Vassallo M, Samani NJ. Rapid Review
of ECG Interpretation. CRC press, Boca Raton: Manson Publishing; 2005. 127 p.
16.
Bachorik PS. Collection of blood samples for
lipoprotein analysis. Clinical chemistry. 1982;28(6):1375-8.
17.
Sperber GH. Clinically Oriented Anatomy. J
Anat. 2006;208(3):393-.
18.
Mannheimer PD. The light-tissue interaction
of pulse oximetry. Anesthesia and analgesia. 2007;105(6 Suppl):S10-S7.
19.
Batchelder PB, Raley DM. Maximizing the
laboratory setting for testing devices and understanding statistical output in
pulse oximetry. Anesthesia and analgesia. 2007;105(6 Suppl):S85-S94.
20.
Brendorp B, Elming H, Jun L, Køber L, Malik
M, Jensen GB, et al. Qtc interval as a guide to select those patients with
congestive heart failure and reduced left ventricular systolic function who
will benefit from antiarrhythmic treatment with dofetilide. Circulation.
2001;103(10):1422-7.
21.
Dahou A, Toubal O, Clavel MA, Beaudoin J, Magne
J, Mathieu P, et al. Relationship Between QT Interval and Outcome in Low‐Flow Low‐Gradient Aortic Stenosis With Low Left
Ventricular Ejection Fraction. Journal of the American Heart
Association.5(10):e003980.
22.
Husby MP, Soliman EZ, Goldberger JJ, Liu K,
Lloyd-Jones D, Durazo-Arvizu R, et al. The Association between the PR Interval
and Left Ventricular Measurements in the Multiethnic Study of Atherosclerosis.
Cardiol Res Pract. 2015;2015:193698-.
23.
Nikolaidou T, Pellicori P, Zhang J, Kazmi S,
Goode KM, Cleland JG, et al. Prevalence, predictors, and prognostic
implications of PR interval prolongation in patients with heart failure. Clin
Res Cardiol. 2018;107(2):108-19.
24.
Akinbami A, Dosunmu A, Adediran A, Oshinaike
O, Phillip A, Vincent O, et al. Steady state hemoglobin concentration and
packed cell volume in homozygous sickle cell disease patients in Lagos,
Nigeria. Caspian J Intern Med. 2012;3(2):405-9.
25.
West MS, Wethers D, Smith J, Steinberg M.
Laboratory profile of sickle cell disease: a cross-sectional analysis. The
Cooperative Study of Sickle Cell Disease. J Clin Epidemiol. 1992;45(8):893-909.
26.
Porter WB, JamesIII GW. The Heart in Anemia.
Circulation. 1953;8:111–6.
27.
Sugimoto T, Sagawa K, Guyton A. Effect of
tachycardia on cardiac output during normal and increased venous return.
American Journal of Physiology-Legacy Content. 1966;211(2):288-92.
28.
Pakkam ML, Brown KN. Physiology, Bainbridge
Reflex. StatPearls. Treasure Island
(FL): StatPearls Publishing LLC.; 2020.
29.
Crystal GJ, Salem MR. The Bainbridge and the
"reverse" Bainbridge reflexes: history, physiology, and clinical
relevance. Anesthesia and analgesia. 2012;114(3):520-32.
30.
Reinier K, Narayanan K, Uy-Evanado A,
Teodorescu C, Chugh H, Mack WJ, et al. Electrocardiographic Markers and the
Left Ventricular Ejection Fraction have Cumulative Effects on Risk of Sudden
Cardiac Death. JACC Clin Electrophysiol. 2015;1(6):542-50.
31.
Grassi G. Heart Rate as Marker of
Cardiovascular Risk. E-Journal of Cardiology Practice. 2007;5(21).
32.
Lang CC, Gupta S, Kalra P, Keavney B, Menown
I, Morley C, et al. Elevated heart rate and cardiovascular outcomes in patients
with coronary artery disease: clinical evidence and pathophysiological
mechanisms. Atherosclerosis. 2010;212(1):1-8.
33.
Hall AS, Palmer S. The heart rate hypothesis:
ready to be tested. Heart. 2008;94(5):561-5.
34.
Böhm M, Schumacher H, Teo KK, Lonn EM, Mahfoud
F, Ukena C, et al. Resting heart rate and cardiovascular outcomes in diabetic
and non-diabetic individuals at high cardiovascular risk analysis from the
ONTARGET/TRANSCEND trials. European heart journal. 2020;41(2):231-8.
35.
Chen X-j, Barywani SB, Hansson P-O, Östgärd
Thunström E, Rosengren A, Ergatoudes C, et al. Impact of changes in heart rate
with age on all-cause death and cardiovascular events in 50-year-old men from
the general population. Open heart. 2019;6(1):e000856.
36.
Tian J, Yuan Y, Shen M, Zhang X, He M, Guo H,
et al. Association of resting heart rate and its change with incident
cardiovascular events in the middle-aged and older Chinese. Scientific Reports.
2019;9(1):6556.
37.
Odesina V. Intravenous support for the
patient in sickle cell crisis. J Intraven Nurs. 2001;24(1):32-7.
38.
Wethers DL. Sickle cell disease in childhood:
Part II. Diagnosis and treatment of major complications and recent advances in
treatment. Am Fam Physician. 2000;62(6):1309-14.
|
Cite this Article: Ikedue MI; Mojiminiyi
FBO; Ndakotsu MA; Isezuo
SA; Bamaiyi AJ (2021). Increased Heart Rate and
Steady State Among Sickle Cell Patients Seen in Sokoto,
North-West Nigeria. Greener Journal of Human Physiology and Anatomy, 3(1): 1-10.
|



