INTRODUCTION
Environmental
pollution through heavy metals has become a universal problem in aquatic
network due to their toxicity, accumulation and bio-magnification (Afshan et
al., 2014). Trace metals in contrast to most pollutants, are not bio
degradable, and they undergo a global ecological cycle in which natural water
are the main pathways. Heavy metals can be concentrated along the food chain,
producing their toxic effect at points far from the source of pollution (Tilzer
and Khondker, 1993). The pollution of aquatic network is a growing threat to
the environment due to agricultural, domestic and industrial contaminants.
Metals make up an important fraction of environmentally hazardous substances.
Nigerian rivers, streams and ponds have received high levels of heavy metals as
a result of the development of industries and anthropogenic activities around
these water bodies (Ahmed et al., 2003; MacFarlane and Burchette 2000; Amisah
et al., 2009).
Heavy metal is any metallic chemical element
that has a relatively high density and is toxic or poisonous at low
concentrations (Ngumbu et al., 2017). Some metals, including chromium
(Cr), lead (Pb), cadmium (Cd), arsenic (As) and mercury (Hg) are known to be very toxic even at low concentrations
(Nguyen et al., 2005), Others, such as copper (Cu), iron (Fe), zinc
(Zn), manganese (Mn) and cobalt (Co) are known to be essential elements which
play important roles in biological metabolism at very low concentrations (Ajmal
et al., 1988; Subotić et al., 2013). However, an excess or
dearth of any of these metals can disrupt biochemical functions in both humans
and animals, at higher concentrations they can lead to poisoning (Lenntech,
2014). The metal which has a relatively high density and toxic at low quantity
is referred as heavy metal, arsenic (As), lead (Pb), mercury (Hg), cadmium
(Cd), chromium (Cr), thallium (TI), etc. fall in this group
Some trace elements are also known as heavy
metals, e.g., copper (Cu), selenium (Se) and zinc (Zn). They are essential to
maintaining the body metabolism, but they are toxic at higher concentrations.
These heavy metals can get to our bodies through food, drinking water and air
(Lenntech, 2014).
These metals are discharged into the
environment through several anthropogenic sources such as the burning of fossil
fuels, transportation, industrial effluent discharge and indiscriminate waste
dumping and are often washed into receiving bodies such as sediment, soil and
water bodies by rain run-off therefore, the aquatic ecosystem receives the bulk
of contaminants from anthropogenic sources (Eletta et al., 2003). These
metals are of particular concern due to their toxic effect at certain levels of
exposure and ability in bio-accumulate in an ecosystem, body tissues and organs
(Babalola, 2010; Eletta et al., 2003).
The occurrence of toxic metals in pond,
stream and river water affects the lives of humans and animals that depend upon
these water sources for their daily life (Khan. 2000). The consumption of
aquatic resources containing toxic metals may cause serious health hazards
through food chain magnification (Khan. 2000; Akan et
al., 2012). Heavy metal intake by fish in polluted aquatic environments
vary and depend on ecological requirements, metabolism and other factors such
as salinity, water pollution level, food and sediment (Adeniyi, 2007; Ajeagah et
al., 2013; Milenković et al., 2005; Vuković et al.,
2014).
The results of many researches show
bioaccumulation of metal pollutants from the water in various tissues of
aquatic organisms (Has-Schön et al., 2006; Monroy et al., 2014;
Zhuang et al., 2013). Various species of fish can be bioindicators of
contamination with heavy metals and others pollutants (Dural et al.,
2007; Huang, 2003, USEPA. 2009: Molina, 2011; FAO, 2012). Feeding habits have a
great influence on pollutants accumulation, especially regarding heavy metals
in different fish species (Amundsen et al.,
1997; Merciai et al., 2014; Abubakar et al., 2015). Many studies
have been done on the levels of heavy metals in fish samples in Africa, Nigeria
and elsewhere (Voegborlo and Akagi, 2007; Voegborlo and
adimado, 2010 and Kwaansa-Ansah, 2012).
In Brass Local Government Area (LGA), limited
research on heavy metals in fish have been
reported. Although, Brass local Government Area is not a heavily industrialize
area, but with the presence of the crude oil exploration companies, it has its
share of pollutants within her environment. Anthropogenic activities such as
crude oil spillage and other chemical waste, domestic wastewater and petrol
from fishing and transport boats have increased over the years, and have led to
very serious environmental pollution within and along the Brass River.
Study area
Brass
is a Local Government Area in Bayelsa State, southern Nigeria. Its headquarter
is in the town of Twon-Brass on Brass Island along the coast, it has a
coastline of approximately 90 km on the Bight of Bonny. It has an area of 1,404
km2 and a population of 185,049 at the 2006 census. It is a traditional town of
the Ijo people, it became a slave-trading port in the early 19th century. It
was ruled by African merchant “houses,” which were encouraged by the European
traders (Gertzel, 1962).
Brass has enormous deposits of crude oil and
natural gas and because of the rich natural resources has the presence of
several national and international oil mining companies. The activities of
these oil mining companies have contributed to the most of the economic development
of the Brass area. In Brass people another economic activity that sustain
livelihood in the area is Fishing and the making of fishing nets, construction
of canoes also is another key economic feature of the Brass people.
The study area is characterized by an
equatorial climate, with a wet season (April to October) and a dry season
(November to March), and with rather constant ambient temperatures (27-34°C)
year-round.
Sample collection
The
three fish samples were randomly collected through the assistance of fisherman
using traps and set nets around the Brass River in Brass Local Government Area
and were immediately transported to the laboratory for analysis. Before the
analysis of the heavy metals’ concentration, the fishes were properly rinsed in
distilled water to remove dirts and debris from the skin and gills
Digestion of Fish
Samples
10g
of each fish were weighed and dried in the hot air oven at 105oC. The
samples were digested with a mixture of HNO3, HClO3 and H2SO4.
These chemicals were mixed in a ratio of 10:4:1. The heavy metals Lead (Pb),
Cadmium (Cd), Chromium (Cr), Hg and As concentrations in the samples were
evaluated using Varian Spectra A100 Atomic Absorption Spectroscopy (AAS) using
prepared standard solution.
Silver Catfish, Chrysichthys
nigrodigitatus
The
silver catfish, Chrysichthys nigrodigitatus (Lacépède,
1803) is among the dominant African commercial fishes of high economic
value and widely serves as food for human consumption in West Africa (Leveque, 1997, Adite and van
Thielen, 1995). This catfish belongs to the genus Chrysichthys,
family Claroteidae, Siluriformes order and Ostariophysi super
order. Synonimies are Pimelodus nigrodigitaus, Arius
acutivelus, Chrysichthys furcatus, Chrysichthys buettikoferi
and Chrysichthys cameronensis (Leveque et
al., 1992). In
general, the species showed grew silvery colour with a white belly and a black
adipose. Chrysichthys nigrodigitatus exhibited a pointed snout slightly
longer than or equal to the width of the mouth and the pre-maxyllary tooth
plate width made 20-30% of the head length (Leveque et al., 1992; Laleye et al., 1995). In the tropical estuary
of Nigeria, Asuquo et al., (1999) the species
as foraging on a variety of benthic food resources. The specie also habits in
salt and fresh water areas in Nigeria.
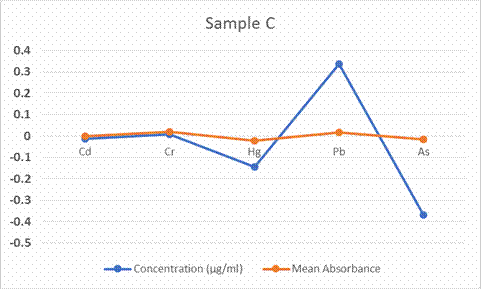
In figure 6, the
result show that the concentration of Pb (0.337µg/ml) and Cr (0.007µg/ml) were
the highest in the Mugil Cephalus with a relative standard deviation of
9.53% and 3.56% respectively. Cd, Hg and As have negative values.
DISCUSSION
The
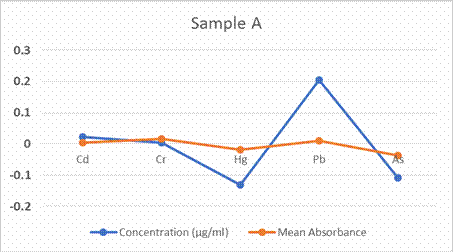
result show that the concentration of Pb (0.204 µg/ml), Cd (0.022 µg/ml) and Cr
(0.004 µg/ml) were the highest in the Chrysichthys nigrodigitatus with a
high Relative Standard Deviation. While Hg and As have negative values. In
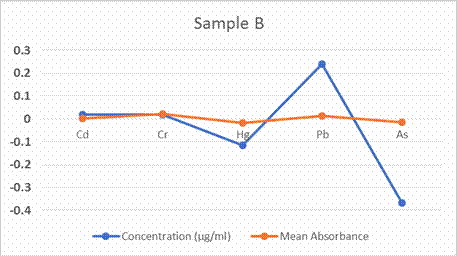
Micropogonias undulatus, the concentration of Pb (0.241µg/ml), Cd
(0.020µg/ml) and Cr (0.018µg/ml) were the highest with a relative standard deviation
of 8.28%, 19.94% and 15.55% respectively. Hg and As have negative values. In Mugil
Cephalus, the concentration of Pb (0.337µg/ml) and Cr (0.007µg/ml) were the
highest with a relative standard deviation of 9.53% and 3.56% respectively. Cd,
Hg and As have negative values. The level of these heavy metals in fishes of
the river were found below the permissible limit.
The heavy metals concentrations were
variously distributed in the fish tissues. The Pb content in three fish muscle
was the highest heavy metal observed in this study. However, the values were
lower than the WHO required concentration in fish (Zrnčić
et al., 2013). The three fish
species, Chrysichthys nigrodigitatus, Micropogonias undulatus and
Mugil cephalus feed on plankton and algae and it can be one of the reasons
for their higher lead bioaccumulation. The variation of accumulation of heavy
metals in different fish species is attributed to several feeding habits.
Significant concentrations of Cd are present in the three fish tissues, The
accumulation of Cd in fish tissues is shown in several studies (Has-Schön et al., 2006; Jarić et al.,
2011; Castro-Gonzalez
et al., 2008). Mercury is one of the very toxic metals with a
tendency to bind to the sulfide group of proteins and deposits in muscles (Castro-Gonzalez et al., 2008) in this study,
Mercury concentration in the fish tissues were found to be negative. But these
values of heavy metal contents are also below the maximum permitted
concentrations of these pollutants according to the Commission of the European
Communities (EC), hence the three fish can be acceptable for human consumption.
However, the heavy metals analysed in this research are known to be very toxic
even at low concentrations (Tešić et al.,
2014). Considering all these results, it is necessary to monitor the
presence of heavy metals as environmental pollutants in the future, thus
ensuring a good ecological status of the Brass River, as well as providing
healthy and safe fresh fish.
CONCLUSION
The
research work reveals the presence of heavy metal concentrations level in the
selected fish species collected from the Brass River. Overall, the heavy metal
concentration level in the fish species were below the standard acceptable
limits which may not pose threat on human health. However, the heavy metals
analysed in this research are known to be very toxic even at low
concentrations. The Brass River should be protected to save the aquatic biota
of the river and more sustainable measures should be taken to ensure better
fish quality and aquatic life of the Brass River. Adequate attention should be
given to ensure that there is no any further pollution of heavy metals in the
river in order to avoid future deleterious health issues to humans.
REFERENCES
Adeniyi
A. A., Yusuf K. A Okedeyi O. O (2007) Assessment of the exposure of two fish
species to metals pollution in the Ogun River catchments, Ketu, Lagos, Nigeria,
Environmental Monitoring and Assessment. 137(1-3):451-8. doi:
10.1007/s10661-007-9780-5.
Adite
A. and Van Thielen R. (1995) Ecology and Fish Catches in the Natural Lakes of
Benin, West Africa. Environmental Biology of Fishes, 43, 381-391. http://dx.doi.org/10.1007/BF00001173
Afshan,
S., Ali, S., Ameen, U. S., Farid, M., Bharwana, S. A., Hannan, F., and Ahmad,
R. (2014) Effect of Different Heavy Metal Pollution on Fish. Research
Journal of Chemical and Environmental Sciences, 2(1): 74-79.
Ahmed
A.T. A, Mandal S., Chowdhury D. A, and Rahman M. M (2012) Bioaccumulation of
Some Heavy Metals in Ayre Fish, Sediment and Water in Dhaleshwari River in dry
Season. Bangladesh Journal of Zoology; 40(1):147-153.
Ajeagah
G., Bikitbe J., and Longo F. (2013) Qualité bioécologique d'un milieu lacustre
hyper-eutrophisé en zone équatoriale (Afrique Centrale): Peuplement de
protozoaires ciliés et macro invertébrés bentho-aquatiques [Bio-ecological
assessment of a hyper-eutrophic lake in an equatorial region (Central Africa):
Population dynamics of ciliated protozoa and bentho-aquatic macro
invertebrates]. Afrique Science. Vol. 9. No. 2 p. 51–53.
Ajmal
M., Khan H. A., Ahmad S. and Ahmad A. (1988) Role of sawdust in the removal of
copper (II) from industrial wastes, Water Research Volume 32, Issue 10,
Pages 3085-3091
Akan J. C, Mohmoud S., Yikala B. S., and
Ogugbuaja V. O (2012) Bioaccumulation of Some Heavy Metals in Fish Samples from
River Benue in Vinikilang, Adamawa State, Nigeria. American Journal of
Analytical Chemistry 3: 727–736.
Amisah,
S., Oteng, M. and Ofori, J. (2009) Growth Performance of the African Catfish,
Clarias gariepinus, Fed Varying Inclusion Levels of Leucaena leucocephala Leaf
Meal. Journal of Applied Sciences and Environmental Management, 13,
21-26. http://www.bioline.org.br/ja https://doi.org/10.4314/jasem.v13i1.55257
Amundsen
P., Staldvik F. J, Lukin A. A, Kashulin N. A, Popova O. A, and Reshetnikov Y.
S. (1997) Heavy metal contamination in freshwater fi sh from the border region
between Norway and Russia. Science of the Total Environment, 201:
211–224.
Arthur, J. R. and Lumanlan-Mayo, S. (1997) Checklist of the
parasites of fishes of the Philippines. FAO Fisheries Technical Paper No. 369.
FAO, Rome, Italy. 102 pp.
Asuquo
F. E., Ogri, O. R. and Bassey, E. S. (1999) Distribution of Heavy Metals and
Total Hydrocarbons in Coastal Waters and Sediments of Cross River State, South
Eastern Nigeria, Internal Journal of Tropical Environment 2, 229-242.
Babalola
Y. T., Babalola A. D. and Okhale F. O (2010) Awareness and Accessibility of
Environmental Information in Nigeria: Evidence from Delta State, Library
Philosophy and Practice (e-journal)
Badran, A. F. (1994) Preliminary
investigations on streptococcosis among freshwater and marine fishes. Veterinary
Medical Journal Giza, 42(1B): 257–262.
Castro-Gonzalez M. I., Mendez-Armenta
M. (2008) Heavy metals: Implications associated to fish consumption. Environmental
Toxicology and Pharmacology, 26:263–271.
Chen, S.C., Liaw, L.L., Su, H.Y., Ko, S.C., Wu,
C.Y., Chaung, H.C., Tsai, Y.H., Yang, K.L., Chen, Y.C., Chen, T.H., Lin, G.R.,
Cheng, S.Y., Lin, Y.D., Lee, J.L., Lai, C.C., Weng Y.J. and Chu, S.Y. (2002) Lactococcus
garvieae, a cause of disease in grey mullet, Mugil cephalus L., in Taiwan.
Journal of Fish Diseases, 25:727–732.
Dural
M., Ziya L. G., and Argun A. Ö. (2007) Investigation of heavy metal levels in
economically important fish species captured from the Tuzla lagoon. Food
Chemistry Volume 102, Issue 1, Pages 415-421
Eletta
O. A., Adekola F. A., and Omotosho J. (2003) Determination of concentration of
heavy metals in two common fish species from Asa River, Ilorin, Nigeria. Toxicology
and Environmental Chemistry. 85(1-3): 7-12.
FAO
(1995) Code of Conduct for Responsible Fisheries. FAO, Rome, Italy. 41 pp.
Food
and Agriculture Organization of the United Nations (FAO) (2012). Harmonized
World Soil Database (Version 1.2). Food Agriculture Organization. http://webarchive.iiasa.ac.at/Research/LUC/External-World-soil-database/HTML/
Froese
R. and Pauly D. (2017) Fish Base. World Wide Web Electronic Publication.
www.fishbase.org
Getzels
J. W., and Jackson P. W. (1962) Creativity and Intelligence: Exploration with
Gifted Students. New York: John Wiley & Sons.
Harrison, I. J. and Senou, H. (1999) Order Mugiliformes.
Mugilidae. Mullets. In: K. E. Carpenter and V. H. Niem (eds.), FAO
species identification guide for fishery purposes. The living marine
resources of the Western Central Pacific. Volume 4. Bony fishes Part 2 (Mugilidae
to Carangidae), pp. 2069–2108. FAO, Rome, Italy.
Has-Schön
E., Bogut I., and Strelec I. (2006) Heavy Metal Profile in Five Fish Species
Included in Human Diet, Domiciled in the End Flow of River Neretva (Croatia). Archives
of Environmental Contamination and Toxicology, 50: 545–551.
Huang
B.W. (2003) Heavy Metal Concentrations in the Common Benthic Fishes Caught from
the Coastal Waters of Eastern Taiwan. Journal of Food and Drug Analysis,
11, 324-330.
Jarić I., Višnjić-Jeftić Ž.,
Cvijanović G., Gačić Z., Jovanović L., Skorić S., and
Lenhardt M. (2011) Determination of differential heavy metal and trace element
accumulation in liver, gills, intestine and muscle of sterlet (Acipenser
ruthenus) from the Danube River in Serbia by ICP-OES. Microchemical Journal,
98: 77–81.
Khan,
A. G., Azim, A., Nadeem, M. A. and Ayaz M. (2000) The effect of formaldehyde
treatment of solvent and mechanical extracted cottonseed meal on the
performance, digestibility and nitrogen balance in lambs. Asian-Australasian
Journal of Animal Sciences, 13 (6): 785-790
Kwaansa-Ansah
E. E., Samuel O. N., and Francis O. (2012) Heavy metals concentration and human
health risk assessmentin seven commercial fish species from Asafo Market,
Ghana, Food Science and Biotechnology 28(2) DOI:
10.1007/s10068-018-0485-z
Lacepède
B. G. E. (1803) Histoire naturelle des poissons. Tome Cinquieme.
5(1-21): i-lxviii + 1-803 + index., available online at
https://www.biodiversitylibrary.org/page/6335629 [details]
Lalèyè, P.,
Philippart J. C. and Heymans J. C. (1995) Cycle annuel de l’indice
gonadosomatique et de la condition chez deux espèces de Chrysichthys
(Siluriformes, Bagridae) du lac Nokoué et de la lagune de Porto-Novo au Bénin.
Cybium. 19:131-142.
Lenntech
(2014) Hazardous Substances. http://www.lenntech.com/hazardous-substances/nitrite.htm
Leveque
C. (1997) Biodiversity Dynamics and Conservation: The Freshwater Fish of
Tropical Africa. Cambridge University Press, Cambridge.
Lévêque,
C., Paugy, D., and Teugels, G. G. (1992). Faune des poissons d’eaux douces et
saumatre de l’Afrique de l’Ouest (521 p.). Tome, 2. Ed. ORSTOM/MRAC.
Macfarlane,
G. B. and Burchett, M. D (2000) Cellular Distribution of Cu, Pb and Zn in the
Grey Mangrove Avicemnia marina (Forsk) Vierh. Aquatic Botany, 68, 45-59.
https://doi.org/10.1016/S0304-3770(00)00105-4.
Merciai R., Guasch H., Kumar A., Sabater S.,
and García-Berthou E. (2014) Trace metal concentration and fish size: Variation
among fi sh species in a Mediterranean river. Ecotoxicology and
Environmental Safety, 107: 154–161.
Milenković
N, Damjanović M, and Ristić M. (2005) Study of Heavy Metal Pollution
in Sediments from the Iron Gate (Danube River), Serbia and Montenegro. Polish
Journal of Environmental Studies 2005, 14: 781–787.
Molina
V. B. (2011) Health Risk Assessment of Heavy Metals Bioaccumulation in Laguna
de Bay Fish Products, Philippines, 14th World Lake Conference, Austin Texas
Monroy
M., Maceda-Veiga A., and Sostoa A. (2014) Metal concentration in water,
sediment and four fish species from Lake Titicaca. Science of the Total
Environment, 478: 233–244.
Ngumbu
R. S., Sumo F. P., Kiazolu J. B. and Humphrey P. S. (2017) Assessment of Lead
and Cadmium Residues in Assorted Vegetables Collected from Markets in Monrovia,
Liberia, International Journal of Scientific Research in Science and
Technology, Volume 3: Issue 1
Nguyen
H. L. Leermakers M., and Elskens M. (2005) Correlations, Partitioning and
Bioaccumulation of Heavy Metals between Different Compartments of Lake Balaton.
Science of the Total Environment, 341, 211-226. https://doi.org/10.1016/j.scitotenv.2004.09.019
Oren, O. H. (1981) Aquaculture of grey
mullets. (International Biological Programme No. 26). Cambridge University
Press, Cambridge, England. 507 pp.
Plumb, J. A. (1999) Edwardsiella
septicaemias. In: P.T.K. Woo & D.W. Bruno (eds.), Fish Diseases and
Disorders, Vol. 3: Viral, Bacterial and Fungal Infections, pp. 479–521. CABI,
New York, USA.
Sperry
T. S., and Thomas P. (1999) Identification of two nuclear androgen receptors in
kelp bass (Paralabrax clathratus) and their binding affinities for xenobiotics:
comparison with Atlantic croaker (Micropogonias undulatus) androgen receptors. Biology
of Reproduction. 61:1152–1161
Subotić
S., Visnjic Z., Spasic S., Hegedis A., Krpo-Ćetković J., and Lenhardt
M. (2013) Distribution and accumulation of elements (As, Cu, Fe, Hg, Mn, and
Zn) in tissues of fish species from different trophic levels in the Danube
River at the confluence with the Sava River (Serbia) Environmental Science
and Pollution Research 20(8) DOI: 10.1007/s11356-013-1522-3
Tešić
M., Baltić M., Teodorović V., Nedić D., Mirilović M.,
Marković R., and Aleksić-Angelis A. (2014) Effects of various meal
compositions on production results, economic performances and fish meat
quality. Acta Veterinaria Belgrade, 64: 338-348.
Tilzer
M. M and Khondker M. (1993) Hypertrophic and polluted freshwater ecosystems:
Ecological basis for water resource management, Department of Botany, Dhaka
University, Bangladesh.
USEPA
(2009) National Primary Drinking Water Regulations. United States Environmental
Protection Agency EPA 816-F-09-004.
Voegborlo
R. B., and Akagi H. (2007) Determination of mercury in fish by cold vapour
atomic absorption spectrometry using an automatic mercury analyzer. Food
Chemistry, 100(2): 853-858.
Voegborlo
R. B and Adimado A. A (2010) Total Mercury Distribution in Different Fish
Species Representing Different Trophic Levels from The Atlantic Coast of Ghana,
Journal of Science and Technology, Vol. 30, No. 1 (2010), pp 1-9
Vuković
D., Vuković Ž., and Stanković S. (2014) The impact of the Danube Iron
Gate Dam on heavy metal storage and sediment flux within the reservoir – Catena,
113: 18–23.
Zhuang
Q., Jason K. K., Hinsby C. and Scott D. B. (2013) Methane emissions from
wetlands: biogeochemical, microbial, and modeling perspectives from local to
global scales, https://doi.org/10.1111/gcb.12131
Zrnčić S., Oraić D.,
Ćaleta M., Mihaljević Ž., Zanella D., and Bilandžić N. (2013)
Biomonitoring of heavy metals in fish from the Danube River. Environmental
Monitoring and Assessment, 185: 1189–1198.